Mind-Eye ConnectionThe October 2024 issue of Review of Optometry focuses on the treatment and management of neuro-ophthalmic disorders. Check out the other articles featured in this issue: |
There are a multitude of optic nerve disorders, and clinicians must be adept at considering not only the most common causes, but also thinking out-of-the-box and digging deeper for answers if the patient’s symptoms are ongoing or unresolved. The goal of this article is to provide the optometrist with clinical symptoms, pathology and treatments for three optic nerve disorders that should not go misdiagnosed. Below, three cases are presented that each showcase a different optic nerve disorder. Case 1 is real, while cases 2 and 3 are hypothetical with representative figures.
Case 1
A 37-year-old Caucasian woman presented to clinic with a report of gradual, painless “glare” in the right eye that she had noticed for one month. No past pertinent ocular or medical findings were present. Upon visiting an optometrist, the patient had a visual acuity of 20/30 OD and 20/20 OS with no history of prior correction required and pinhole no improvement OD. Confrontation fields were full to finger counting, slit lamp exam was unremarkable and dilated fundus examination revealed an unremarkable fundus with no pallor, optic disc edema or hemorrhages. Pupils were ERRLA with no afferent pupillary defect (APD). Color vision was normal by Ishihara testing. The patient was referred to a general ophthalmologist, who again found no remarkable findings upon a comprehensive eye examination, thus the ophthalmologist chose to refer the woman to a neuro-ophthalmologist.
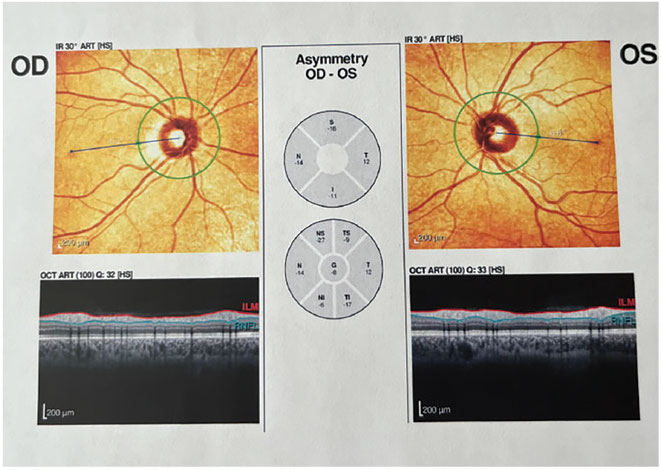 |
|
Fig. 1. OCT with no abnormalities present. Click image to enlarge. |
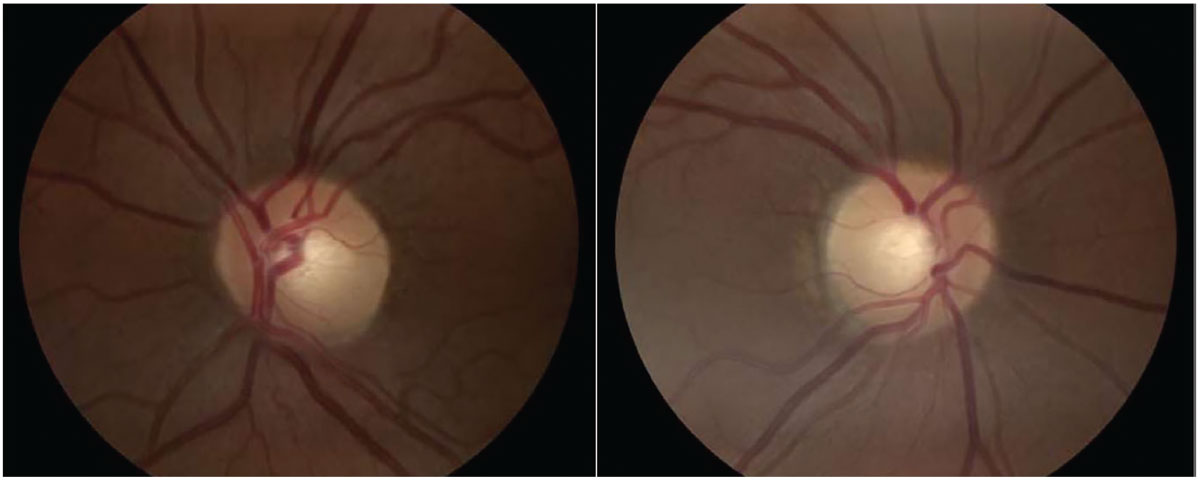 |
|
Fig. 2. Optic nerve head images with no abnormalities present. Click image to enlarge. |
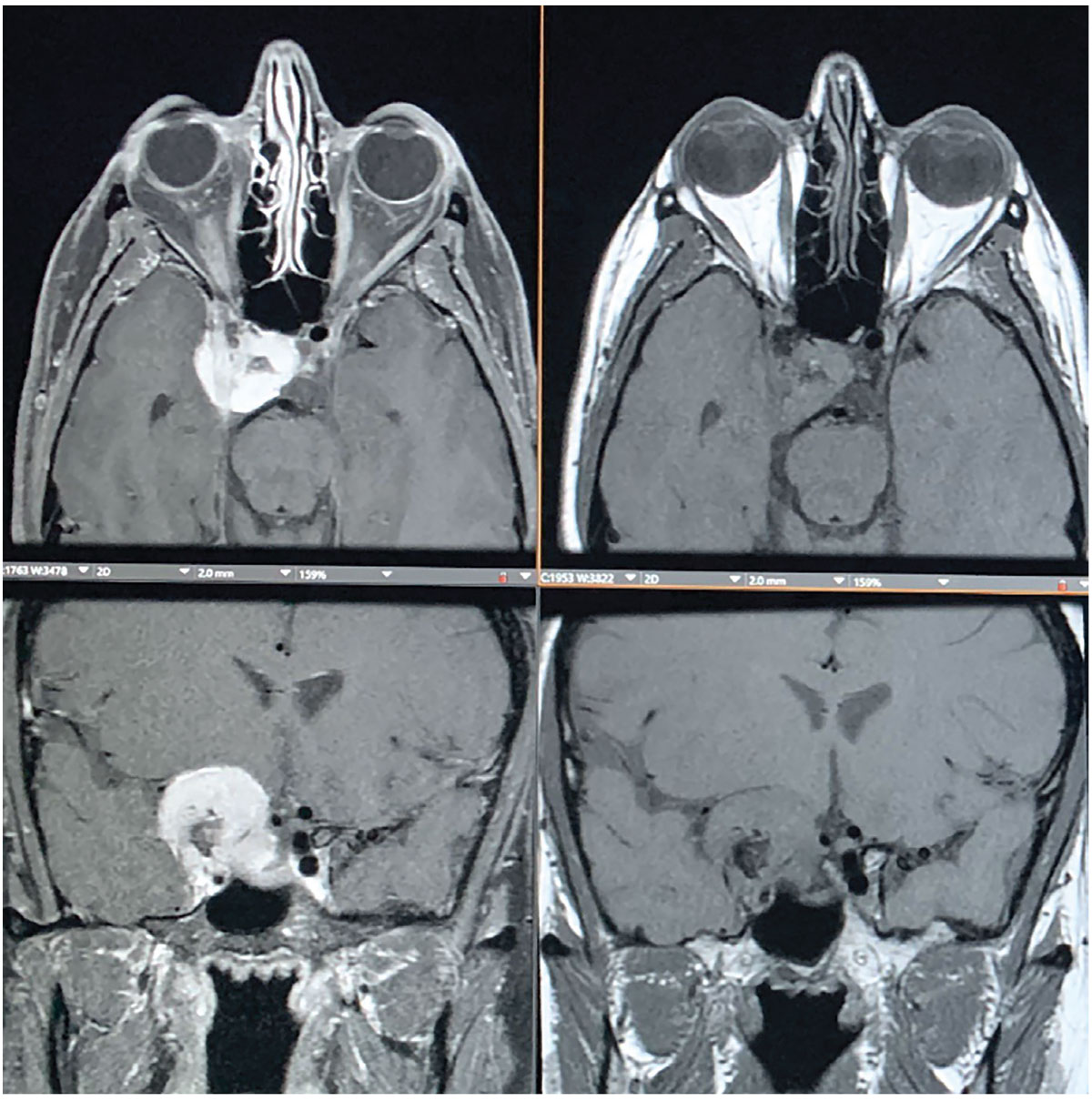 |
|
Fig. 3. MRI shows an avidly enhancing extra-axial mass centered along the right anterior clinoid process, also with a medial displacement of the right optic tract with narrowing and encasement of right optic nerve. The mass extends to the right aspect of the sella turcica and extends inferiorly into the right cavernous sinus. Click image to enlarge. |
Compressive and Infiltrative Optic Neuropathies
Many disorders can occur within the optic nerve, the optic canal or intracranially to compress the optic nerve.1 Compressive optic nerve disorders include orbital tumors, orbital infection, orbital inflammation, intracranial tumors, aneurysm and thyroid eye disease.2 Hallmark symptomatology of optic neuropathy includes progressive, painless vision loss with a relative APD and dyschromatopsia.2
One cause of anterior optic neuropathy is optic nerve sheath meningioma (ONSM). This disorder arises from the intraorbital optic nerve sheath and grows around the optic nerve, ultimately disrupting plial blood supply and axonal transport to the optic nerve.3 ONSMs constitute one-third of all optic nerve tumors and are the second most common optic nerve tumor after glioma.
Mean age upon presentation is 40.8 years old and affects women more commonly than men, with presentation typically unilateral.3 Common presenting symptoms are progressive slow vision loss, double vision and any type of visual field defect.1 Neuroimaging is required for the diagnosis, but typically a biopsy is not.1 Radiology reports with MRI T1-weighted images with contrast will reveal enlargement and enhancement of the optic nerve sheath. Treatment of the disorder will vary based on severity and vision loss, and is still controversial in approach.2,4 Although some patients with minimal vision loss can be observed, fractionated stereotactic radiotherapy can improve vision with minimal consequence.2 This was confirmed by a study that found radiotherapy treatment for ONSMs resulted in good control rates and favorable preservation of vision.5
As discussed in case 1, a common cause of retrobulbar optic neuropathy is a brain meningioma. Typically occurring in Caucasian women of middle age, these brain tumors result in unilateral vision loss. Meningiomas are most common in patients 60 to 70 years of age, with increasing risk with age. They are twice as common in women as men and are the most prevalent benign intracranial tumor, accounting for 13% to 20% of all primary intracranial tumors.6
Anatomically, these tumors arise from the dural coverings of the brain. They are located within either the intraorbital space, optic canal or intracranial space. Vision loss is determined by the location and size of this compressive lesion. Some meningiomas impinge on the optic nerve; some may encase the optic nerve to varying degrees and others can grow entirely in the optic nerve sheath.7 Lesions in the orbit, tuberculum sellae and sphenoid wing can cause symptoms of headache, vision loss, eye pain and proptosis.8
Slow-growing meningioma tumors are typically WHO Grade I, indicating benign status, about 90% of the time. Treatment for these meningiomas is likely surgical, involving gross total resection when anatomically possible. Other potential treatment options include observation when the tumor is not compressing a vital entity nor causing any symptoms, or, alternatively, radiotherapy. Surgical treatment resulted in improvement of visual acuity in 61% of patients with preoperative monocular visual dysfunction.9
Clinical pearl. Upon noting a relative APD in a patient with an otherwise normal fundus and optic nerve, and who has a complaint of progressively dimming vision, compression must be ruled out by neuroimaging, preferably with gadolinium-enhanced MRI.1 Visual field testing is also an essential task to perform in patients who complain of vision loss or vision change.
Case 2
A 22-year-old Caucasian woman with a history of migraine and hypertension presented with a subacute onset of vision loss in her left eye for the past several days, with pain on eye movement. There was no past medical history and she denied any other neurological symptoms. Her visual acuity was 20/20 OD and 20/150 OS with her habitual spectacles. Pupils revealed a left APD, Ishihara color plates revealed a defect in the left eye and the slit lamp and dilated funduscopic examination, including the optic nerve, were unremarkable (Figure 4). Humphrey visual fields were then obtained and were also found unremarkable (Figure 5). T2-weighted and fluid-attenuated inversion recovery sequence MRI of the orbits was also obtained (Figure 6), which confirmed the presence of white matter lesions and plaques in the white matter of the central nervous system; these are hallmark features of multiple sclerosis (MS).10
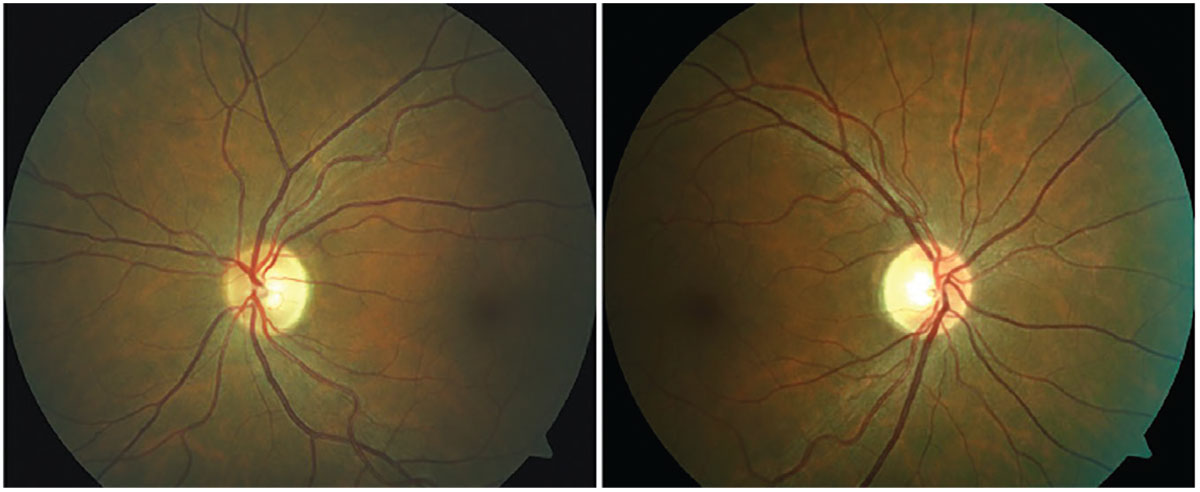 |
|
Fig. 4. Asymptomatic bitemporal optic disc pallor representing progressive demyelination of the optic nerve without a history of optic neuritis. Photo: Michael DelGiodice, OD. Click image to enlarge. |
 |
|
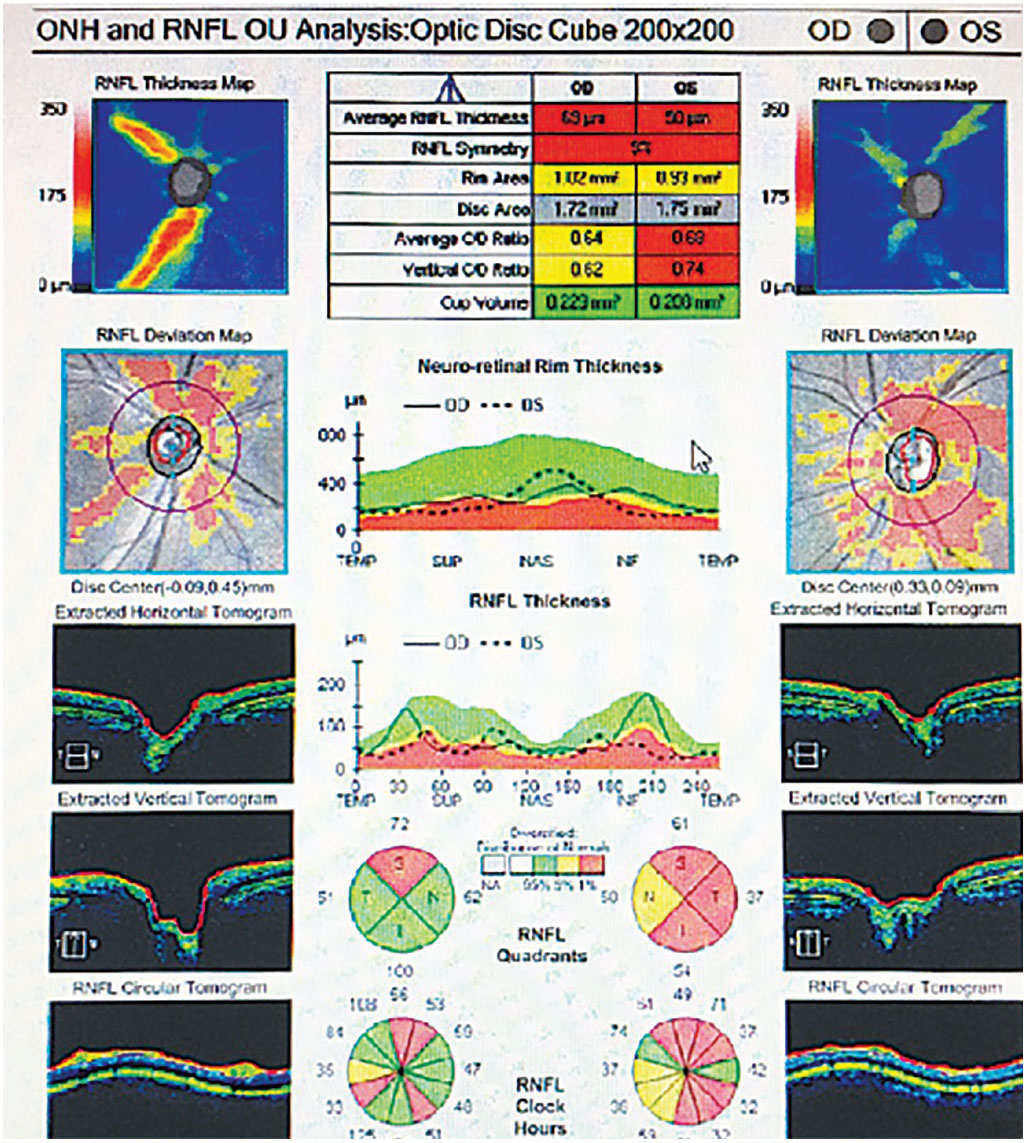
Fig. 5. Bilateral retinal nerve fiber loss OU in a patient with MS without a history of optic neuritis. Photo: Michael DelGiodice, OD. Click image to enlarge. |
 |
|
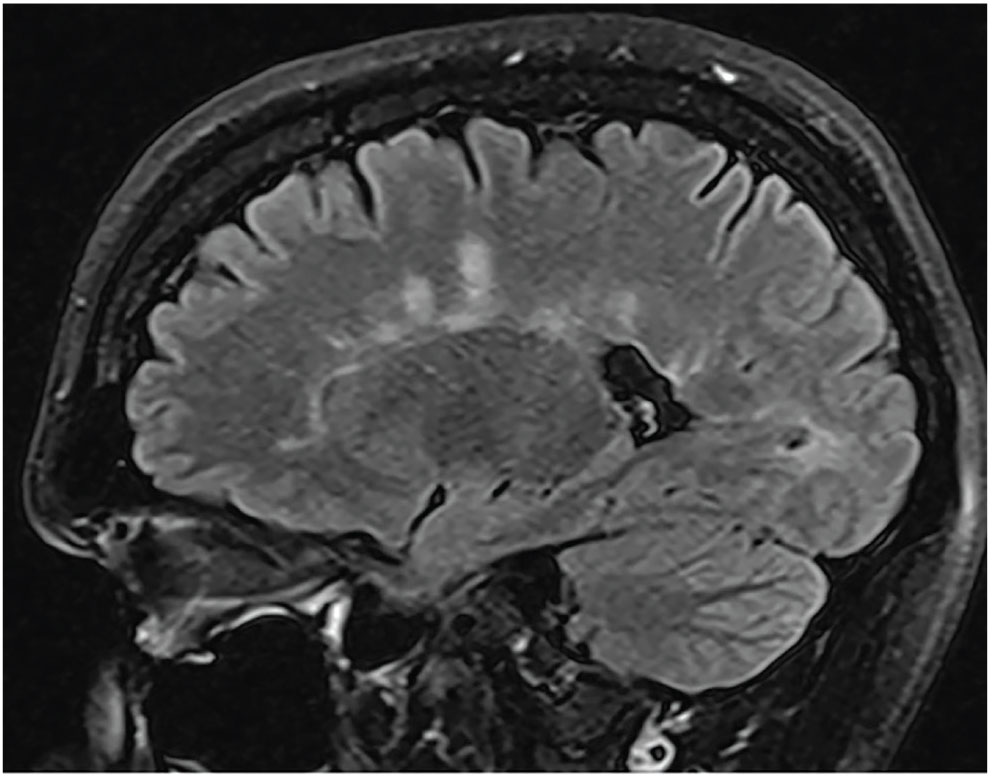
Fig. 6. This patient with right retrobulbar optic neuritis presented with subacute vision loss and pain on eye movement. An MRI brain scan revealed periventricular white matter lesions suggestive of a demyelinating disease. Photo: Alison Bozung, OD. Click image to enlarge. |
Optic Neuritis
The most frequent cause of acute-onset optic neuropathy in young adults is optic neuritis, which can be divided into two categories: retrobulbar optic neuritis (the most common type with minimal optic disc edema) and papillitis (less common but presenting with optic disc edema).11 Although this can be idiopathic, it can have other causes as well, including demyelinating diseases like MS, infections, autoimmune disorders, inflammatory disease and vaccination reactions.11 Some clinicians prefer to categorize optic neuritis as typical (MS) and atypical (non-MS-related causes). There have also been multiple studies finding optic neuritis to occur after COVID-19 vaccination.12
It is essential to consider that optic neuritis is the presenting symptom of MS in 25% of cases and, of all MS cases, optic neuritis will occur in 70% of patients.13 In the Optic Neuritis Treatment Trial (ONTT), MS occurred after 15 years in patients with an abnormal brain scan following a case of optic neuritis as compared to 25% of patients with a normal scan. Most patients that have optic neuritis and will develop MS do so within seven years of initial diagnosis.14 Optic neuritis tends to have a female-to-male predilection of 2:1 and occurs most commonly in patients of Northern European descent, in temperate climates and during springtime, most commonly affecting patients aged 16 to 55.13
Clinical presentation includes loss of central vision, which is the major symptom in more than 90% of patients with acute optic neuritis.14 Visual acuity at onset can vary from 20/20 to light perception. Pain with eye movement, although mild, is typically present in most patients and resolves within days. Less commonly, phosphenes and loss of depth perception can occur. Other symptoms of optic neuritis include Uhthoff’s phenomenon (worsening of vision caused by changes in body temperature) and low contrast sensitivity.13
In the ONTT, visual field defects presented in a variety of fashions and were therefore not helpful in the differentiation of optic neuritis with other optic nerve disorders.14 As well, OCT’s inner retinal measurements during the first month of onset were not predictive of final outcome.15
Recovery is generally good, although it typically begins rapidly and then can continue gradually up to a year after onset. A favorable 20/40 visual acuity is achieved in 90% of patients.14 Treatment of optic neuritis typically consists of IV methylprednisone. According to the ONTT, visual acuity improved to 20/25 after four days of this treatment, in contrast with 15 days of either no treatment or oral steroids.14 IV methylprednisone also improved the six-month outcome of color function and contrast sensitivity. IV methylprednisone (1g daily for three days) followed by a 14-day treatment with oral prednisone (1mg/kg daily) also decreased the two-year rate development of MS. Although helpful in this regard, IV methylprednisone does not improve the ultimate visual outcome.14
Clinical pearl. A typical case of optic neuritis presents as a young adult woman with unilateral pain on eye movement, a normal or swollen optic disc, a relative APD and possible reduced color vision. It is essential to order an MRI of the brain and orbits in all cases of acute optic neuritis to scan for white matter changes. An MRI of the spine is also necessary.16
Case 3
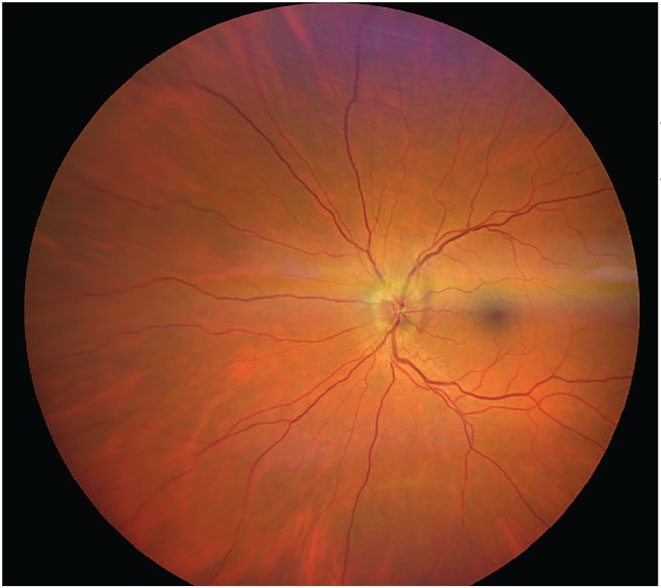 |
|
Fig. 7. NAION presented in the left eye. Photo: Carolyn Majcher, OD. Click image to enlarge. |
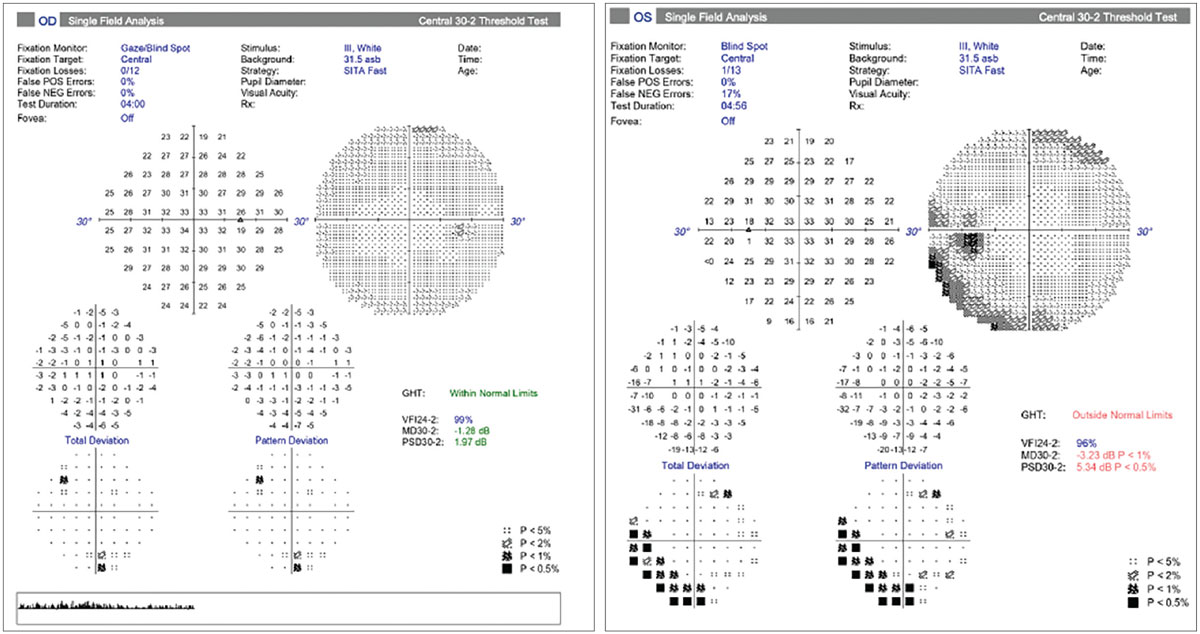 |
|
Fig. 8. Visual fields in NAION. Normal field OD and an inferior altitudinal defect OS. Photo: Carolyn Majcher, OD. Click image to enlarge. |
NAION
The most frequent cause of acute-onset optic neuropathy in adults over 50 years in the Western world is NAION, which is also the second most frequent form of optic neuritis, following glaucoma.17,18 This disease affects patients of European descent, with a 95% predilection in the US; men are more commonly affected than women.17,18
The accepted cause of this disorder is due to ischemia and hypoperfusion of the optic nerve head.17,18 It is supposed that transient or permanent hypoperfusion of the short posterior ciliary arteries that supply the optic nerve head may induce optic disc edema that could incite compartment syndrome in a non-expansible region—the site between the optic nerve head surface and the lamina cribrosa—especially in structurally predisposed crowded optic discs.18
Because 70% of patients awaken with this disorder, it has been linked to nocturnal hypotension and also sleep apnea.18 An important risk factor is the “disc at risk,” which is a small, structurally congested optic disc with a small or absent physiological cup. Disc at risk is present in 25% of all NAION cases. In addition, various medications have been linked to NAION, such as amiodarone and phosphodiesterase type-5 inhibitors (e.g., sildenafil). One study found the most common risk factors for NAION were, in order, cup-to-disc ratio equal to or less than 0.3, diabetes, hyperlipidemia and hypertension.19
As in case 3, the clinical presentation of NAION is typically a rapid onset of monocular visual loss that worsens over a number of days in the presence of a relative APD and optic disc edema. Visual acuity loss is typically unilateral and remains stable in two-thirds of patients. A visual field defect is always observable upon presentation and is described by patients as blurring in the affected visual field region.18 The most common field defect is relative inferior altitudinal with an absolute inferior nasal defect (as demonstrated in Figure 8 for the patient in case 3).6
Optic disc swelling is always present at the onset and is part of the clinical definition of this disorder. Swelling is more commonly diffuse than segmented and the superior portion of the optic nerve is more commonly affected.3 Peripapillary retinal hemorrhages are present in 75% of patients. Color vision loss in this condition is similar and in proportion to the visual acuity loss. In contrast, optic neuritis has a color vision loss much greater than visual acuity loss. NAION patients also do not present with pain on eye movement. A recent study published in June 2023 distinguished a successful deep learning system for differentiating NAION from demyelinating optic neuritis through use of fundus photographs.20
No treatment has been definitively shown to be effective for NAION; however, various options have been presented, such as optic nerve sheath decompression, corticosteroids or aspirin use. Patients should be warned about the risk of developing NAION in the fellow eye. This possibility is clinically significant at 15% to 20% over five years, and mean involvement time of the fellow eye occurs at 2.9 years.3 NAION has also been linked to conditions of optic disc drusen, chlamydia, coagulopathies, migraine and sleep apnea.3
Clinical pearl. In patients suspected of NAION, it is essential to rule out arteritic ischemic optic neuropathy (AION), which is a medical emergency due to potential risks of blindness, stroke and death. AION presents more frequently in patients over 60 and also commonly includes jaw claudication, scalp tenderness and headache. Clinical signs include a swollen, chalky white optic disc. To rule out AAION and giant cell arteritis (GCA), bloodwork with erythrocyte sedimentation rate and C-reactive protein is over 97% specific for detection of GCA. The gold standard of diagnosis for GCA is a temporal artery biopsy.18
Takeaways
A variety of disorders can affect the optic nerve. With the presentation here of one uncommon and two common causes of optic nerve disease, the optometric clinician will be more adept at diagnosing, managing and ultimately treating these important disorders.
Dr. Setork graduated from the Illinois College of Optometry in 2011. She has excelled in specialty contact lenses, pioneered and coordinated meetings for low vision support groups and practiced ocular disease management and comprehensive optometry in both corporate and medical ophthalmology settings.
1. Miller NR, Subramanian PS, Patel VR (eds). Walsh and Hoyt’s clinical neuro-ophthalmology: the essentials. 3rd ed. Wolters Kluwer. 2016. 2. Thurtell MJ, Tomsak RL. Compressive optic neuropathy. Neuro-ophthalmology (what do I do now). 2nd ed. Oxford University Press. 2019:21. 3. Thurtell MJ, Tomsak RL. Arteritic ischemic optic neuropathy. Neuro-ophthalmology (what do I do now). 2nd ed. Oxford University Press. 2019:9-14. 4. Marchetti M, Bianchi S, Milanesi I, et al. Multisession radiosurgery for optic nerve sheath meningiomas—an effective option: preliminary results of a single-center experience. Neurosurgery. 2011;69(5):1116-22; discussion 1122-3. 5. Smee RI, Schneider M, Williams JR. Optic nerve sheath meningiomas—non-surgical treatment. Clin Oncol (R Coll Radiol). 2009;21(1):8-13. 6. Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. Lancet. 2004;363(9420):1535-43. 7. Hénaux PL, Bretonnier M, Le Reste PJ, Morandi X. Modern management of meningiomas compressing the optic nerve: a systematic review. World Neurosurg. 2018;118:e677-86. 8. Parish JM, Shields M, Jones M, Wait SD, Deshmukh VR. Proptosis, orbital pain, and long-standing monocular vision loss resolved by surgical resection of intraosseous spheno-orbital meningioma: a case report and literature review. J Neurol Surg Rep. 2020;81(1):e28-32. 9. Zhang Y, Kim J, Andrews C, et al. Visual outcomes in surgically treated intracranial meningiomas. J Neuroophthalmol. 2021;41(4):e548-59. 10. Cairns J, Vavasour IM, Traboulsee A, et al. Diffusely abnormal white matter in multiple sclerosis. J Neuroimaging. 2022;32(1):5-16. 11. Thurtell MJ, Tomsak RL. Optic neuritis. Neuro-ophthalmology (what do I do now). 2nd ed. Oxford University Press. 2019:3-19. 12. Lee WJA. COVID-19 vaccine-associated optic neuritis. QJM. 2022;115(10):683-5. 13. Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol. 2014;13(1):83-99. 14. Chan JW. Optic neuritis. Optic nerve disorders: diagnosis and management. 1st ed. Springer. 2007:1-30. 15. Deschamps R, Shor N, Vignal C, et al. Prospective longitudinal study on prognostic factors of visual recovery and structural change after a first episode of optic neuritis. Eur J Neurol. 2022;29(9):2781-91. 16. Bajpai V, Madan S, Beri S. Arteritic anterior ischaemic optic neuropathy: an update. Eur J Ophthalmol. 2021;31(6):2818-27. 17. Thurtell MJ, Tomsak RL. Non-arteritic ischemic optic neuropathy. Neuro-ophthalmology (what do I do now). 2nd ed. Oxford University Press. 2019:15-20. 18. Salvetat ML, Pellegrini F, Spadea L, Salati C, Zeppieri M. Non-arteritic anterior ischemic optic neuropathy (NA-AION): a comprehensive overview. Vision (Basel). 2023;7(4):72. 19. Behbehani R, Ali A, Al-Moosa A. Risk factors and visual outcome of non-arteritic ischemic optic neuropathy (NAION): experience of a tertiary center in Kuwait. PLoS One. 2021;16(2):e0247126. 20. Liu K, Liu S, Tan X, et al. Deep learning system for distinguishing optic neuritis from non-arteritic anterior ischemic optic neuropathy at acute phase based on fundus photographs. Front Med (Lausanne). 2023;10:1188542. |

