Mind-Eye ConnectionThe October 2024 issue of Review of Optometry focuses on the treatment and management of neuro-ophthalmic disorders. Check out the other articles featured in this issue: |
Patients with neuro-ophthalmic disorders are a unique subset that harbors potentially life-threatening and sight-threatening conditions. Recognition of these disorders and urgent, proper assessment are vital in optimizing outcomes. Neuro-ophthalmologists and neurologists familiar with the visual system frequently have limited availability, and these vital resources are often not options in urgent situations. Also, referral to a hospital emergency room (ER) with no guidance to the staffing physicians often results in incorrect or delayed diagnoses.
Thus, it is incumbent upon primary eyecare practitioners to be involved, to the extent of personal comfort, in the diagnosis and management of patients with neuro-ophthalmic disorders. Doing so involves knowledge and familiarity with the most common types of conditions, an ability and comfort with ordering neuroimaging and blood work, and a willingness to collaborate with other professionals. Let’s walk through several neuro-ophthalmic disease patients seen in a clinical practice and demonstrate how they can be effectively managed.
Case 1
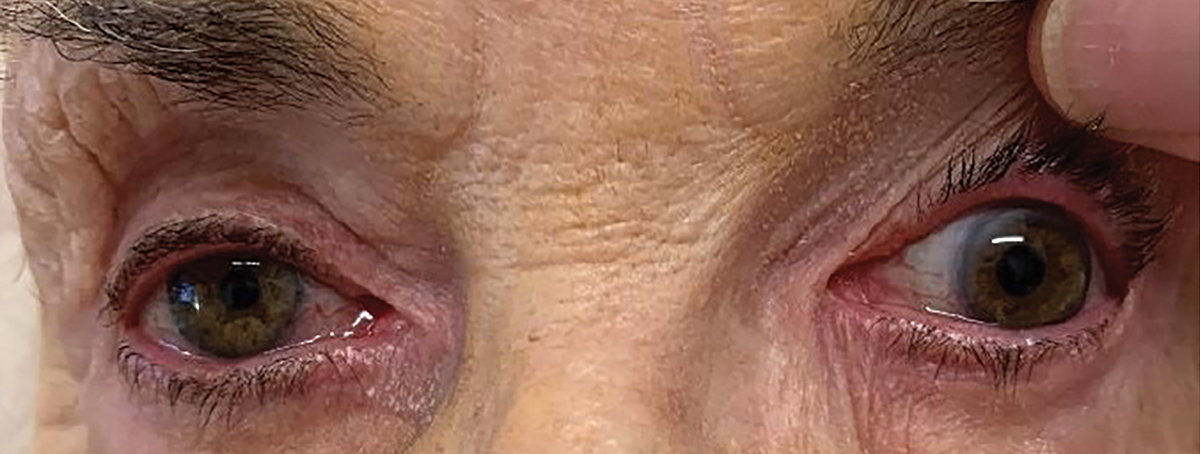
This patient was an 86-year-old woman complaining of eyelid droop and retro-orbital pain who was referred to me urgently from an optometric colleague. Her condition had developed and worsened over a one-week period before she sought care. Upon examination, she had a complete ptosis of her upper left eyelid. Upon raising her eyelid manually, she reported diplopia with a complete loss of adduction, elevation and depression of the eye (Figure 1). Pupil testing showed 3mm of anisocoria in normal room illumination, and there was no light reactivity of the larger left pupil. The diagnosis was a complete cranial nerve (CN) III palsy with pupil involvement, with the likely cause being compression from a posterior communicating aneurysm.
 |
|
Fig. 1. Pupil-involved left CN III palsy in case 1. Click image to enlarge. |
A patient with acute CN III palsy will present with an onset of rapidly unilateral ptosis and ophthalmoplegia. Eye or head pain may be present, dependent upon the cause.1-3 The patient often complains of double vision, though diplopia may be masked by the ptosis. The involved eye in a complete ptosis will be down and out, with limitations of elevation, depression and adduction due to underaction of the superior, inferior and medial recti muscles and inferior oblique muscle.1-4 The underaction of these muscles may be complete or incomplete. In any case of CN III palsy, the pupil may be dilated and minimally reactive to light (pupillary involvement), totally reactive, (pupillary noninvolvement) or may be sluggishly responsive (partial pupillary involvement).1,2
The main concern in CN III palsy is compression of the nerve by an expanding aneurysm of the posterior communicating artery or other adjacent vessel, such as the internal carotid, basilar, anterior communicating or temporal arteries.4-7 Aneurysmal compression is marked by head or retro-orbital pain and anisocoria with ipsilateral pupil dilation and poor light response, as the expanding aneurysm compresses the pupillomotor fibers traveling with CN III as well as pain sensitive dura and other such structures. Vasculopathic palsies may have pain or be painless and pupil size and function are typically normal.
 |
|
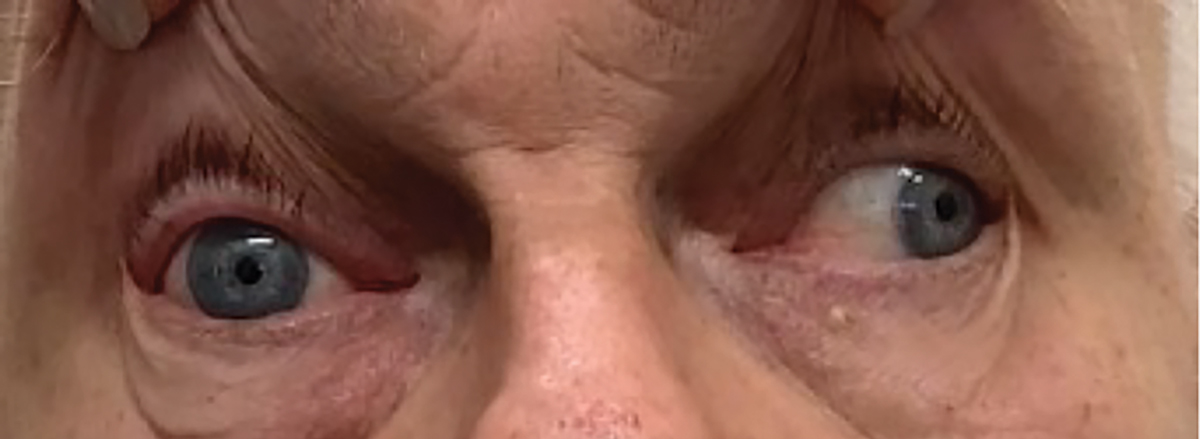
Fig. 2. Pupil sparing vasculopathic CN III palsy in case 2. Click image to enlarge. |
When encountering any patient with either partial or complete CN III palsy, the likelihood of a potentially fatal aneurysm must be assessed. Incomplete palsies (regardless of pupil function) and any with pupil involvement must be critically suspected of having an aneurysm and managed urgently. Appropriate neuroimaging should include CT and CTA or MRI and MRA.8 When the likelihood of an aneurysm is high, as was with this patient, this is best done through an ER with information on the suspected cause and needed testing shared with the managing ER physician. I always call them to detail the situation and share information. I also make sure that the comanaging physician has my direct contact information should any questions or issues arise.
In this situation, I called the ER of the local hospital and spoke with the triage nurse first and then the physician on the floor to describe the patient, her diagnosis, potential for a posterior communicating aneurysm, the appropriate testing needed, as well as the need for a neurosurgical consult immediately. The ER physician thanked me for the information and indicated that he was ready to immediately receive her. She made it promptly to the hospital, where she received the requisite diagnostic testing and care for an aneurysm and has since recovered.
Case 2
Compare and contrast that first patient with a second one having a similar diagnosis. He was a 70-year-old-man who developed left retro-orbital pain, followed by progressive diplopia and ptosis over one week prior to presenting. He also had a complete CN III palsy but was isocoric in the light and dark and had symmetrical pupil light reaction in each eye (Figure 2). His medical history was significant for long-standing type 2 diabetes and hypertension.
 |
|
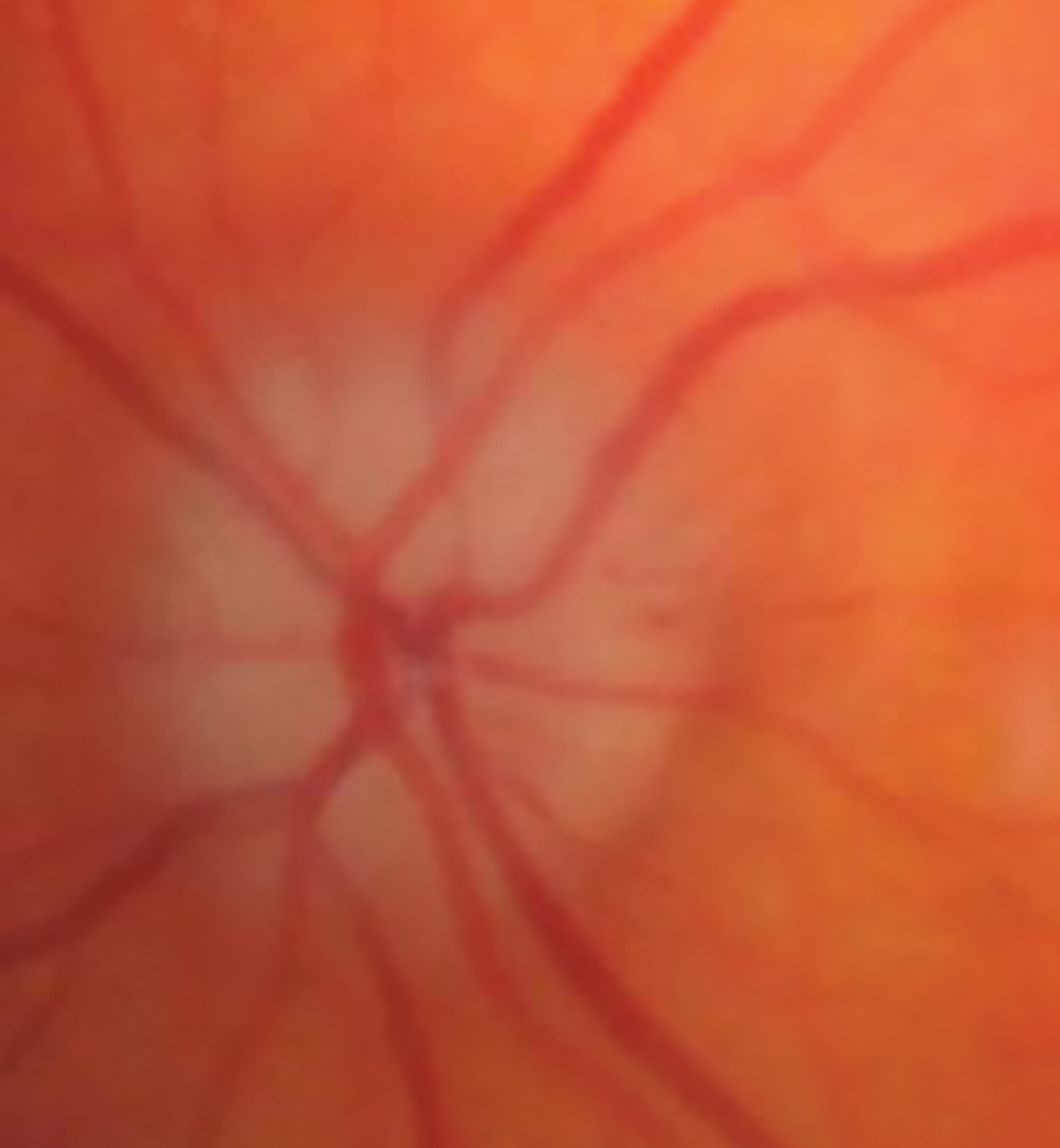
Fig. 3. AAION in this case 3’s left eye. Click image to enlarge. |
Based upon the clinical appearance, the etiology was presumed to be microvascular ischemia and not an aneurysm. Prevailing thoughts are that every patient with a CN III palsy should receive neuroimaging. His CT/CTA, obtained on an outpatient basis the next day, were both normal. He showed improvement in ptosis and motility at six weeks and resolved completely at 12 weeks, consistent with a microvascular palsy.
Case 3
This patient came as a call from an ER physician requesting a consult. The patient was a 64-year-old woman who had woken up completely blind in her left eye. She presented to the ER, where she reported that her vision had improved but was still markedly abnormal. The ER physician noted that she had a relative afferent pupillary defect (RAPD) in her left eye and sought consultation. I agreed to see her immediately.
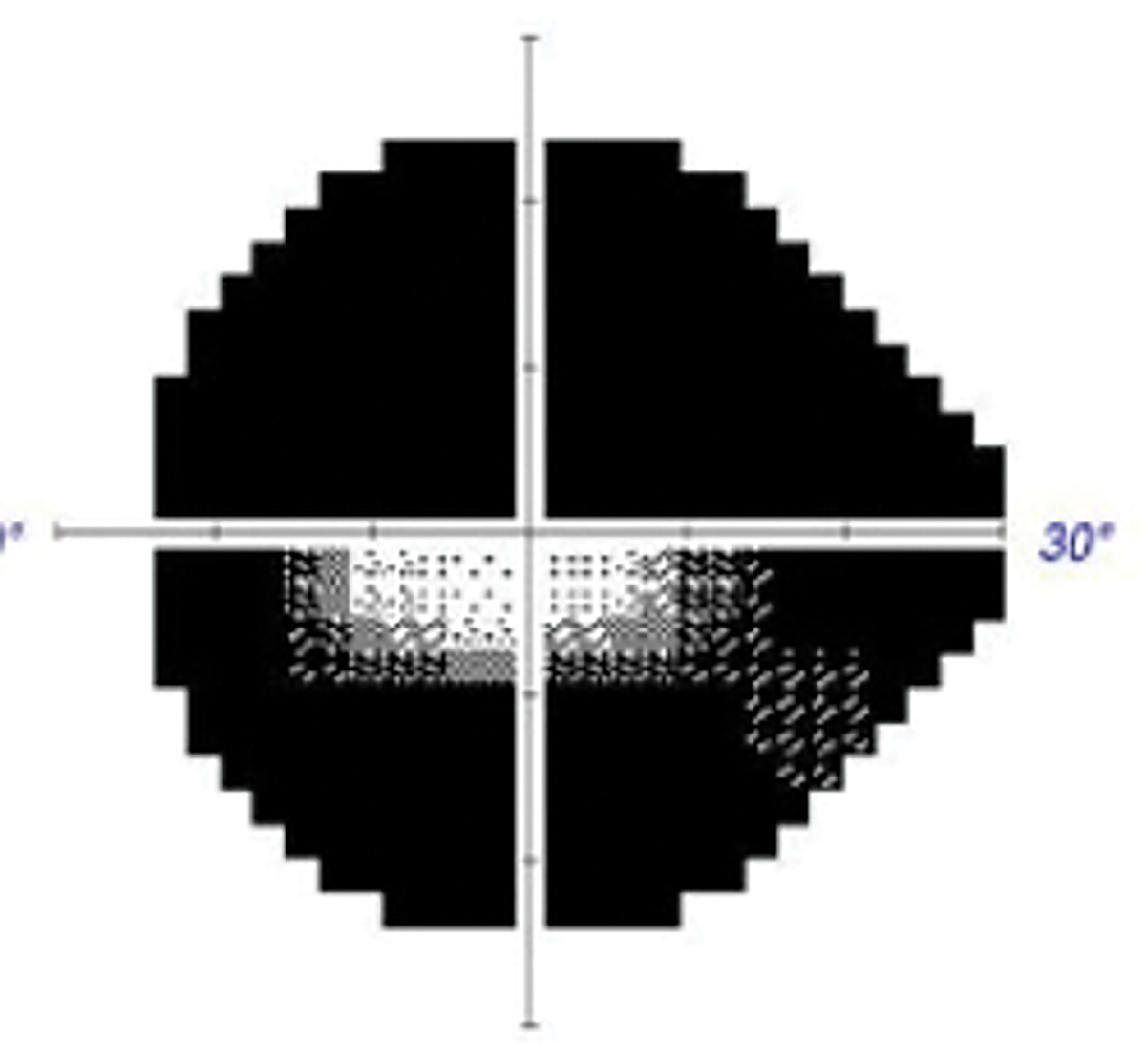
When she arrived 20 minutes later, visual acuity (VA) in her left eye was 20/30, but there was a markedly constricted visual field. She also had a pale and swollen optic disc in that eye (Figures 3 and 4). Upon questioning, she revealed weight loss of 15lbs, frontal/occipital headache and malaise over the past several weeks that she had attributed to a previous COVID infection.
At this point, the suspected diagnosis was an arteritic anterior ischemic optic neuropathy (AAION) from giant cell arteritis (GCA). I educated the patient and called the ER physician with my impressions. I said that I was sending her back with recommendations to evaluate her urgently for GCA with erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and platelet levels and gave him my contact if he had any questions. Several hours later, he called back to report that her preliminary assessment had her ESR markedly elevated at 96mm and asked me what steps should be taken. I recommended that he admit her for 250mg IV solumedrol q6h x three days (12 doses) followed by 80mg oral prednisone until she could be seen by rheumatology and that a temporal artery biopsy or ultrasound should be obtained during her admission. About two hours later, the hospitalist who was going to admit the patient called to reconfirm my recommendations.
AAION is caused by infarction of the short posterior ciliary arteries supplying the anterior optic nerve. Patients with this condition will usually present with a prodrome of anorexia, weight loss, decreased appetite (all due to discomfort while eating from jaw claudication), fever and malaise. The involved optic disc will be swollen, edematous, pale and atrophic, often with associated splinter hemorrhages. Typically, following the event of acute vision loss, the patient will test positively for GCA.9
 |
|
Fig. 4. Accompanying visual field defect in case 3. Click image to enlarge. |
This condition is generally considered after the age of 50. The mean age is 71, with an increasing incidence with advancing age.10 There is a two-to-one female-to-male ratio and a higher incidence in Caucasian patients.10 GCA is a granulomatous inflammation of medium- and large-sized arteries. As virtually any vessel within the body may be involved, this is a multi-system, multi-symptom disorder. The degree of ischemia tolerated varies by system, and there is often a symptomatic period of weeks to months before diagnosis is reached. In the eye, ischemia is manifested often by transient ischemic attacks and intermittent diplopia and ophthalmoplegia prior to complete occlusion of the posterior ciliary, retinal or ophthalmic arteries.11 When symptoms manifest ocularly, there is a much shorter time interval to severe permanent vision loss.
Management begins with the recognition that GCA may be a potential cause of the aforementioned findings in an elderly patient. There is a strong association between GCA and polymyalgia rheumatica (PMR), a rheumatic disorder characterized by pain and stiffness around the neck, shoulder and hip area. The two are related conditions, with some people having symptoms of both. A small percentage of patients with PMR will have GCA, and about half of patients with GCA will have PMR diagnosed. Once GCA is recognized as a potential etiopathology, immediate ESR, platelet count and CRP must be ordered. If these tests are elevated, or if there are obvious constitutional symptoms, then a temporal artery biopsy should be performed in order to conclusively diagnose GCA. Alternately, temporal artery ultrasound has been used increasingly with similar diagnostic ability compared with temporal artery biopsy.12
If the patient is either suspected to have, or is diagnosed with AAION, then systemic steroids must be initiated immediately to prevent vision loss progressing to the other eye. Steroid therapy should not be withheld pending the biopsy or ultrasound. Consensus recommends immediate therapy involves hospital admission with 1g to 2g IV methylprednisolone for two to three days, followed by oral steroids (60mg to 100mg qd of prednisone) with taper as the disease is controlled.13
 |
|
Fig. 5. Optic discs of the patient in case 5. Click image to enlarge. |
Case 4
Compare and contrast that with this patient, a 70-year-old man who initially complained of intermittent horizontal diplopia, which was first ascribed to a decompensating phoria. His diplopia worsened, and he was seen to now manifest a subtle right abduction deficit. He additionally complained of retro-orbital pain. At this point, he had been suspected of having a microvascular CN VI palsy and referred to me for ongoing evaluation. His medical history indicated treated hypertension, and he was not diabetic. I had anticipated significant worsening throughout the early course. However, when I saw him, he felt that the diplopia was not as severe and was now more intermittent than it had been the previous week, which did not follow the typical multi-week course of a microvascular cranial nerve palsy.
Upon detailed questioning, he now described his discomfort as a generalized headache more than a retro-orbital discomfort. He denied weight loss, loss of appetite and jaw claudication. I ordered an urgent ESR, CRP and platelet count at a commercial lab. Results were obtained the next day, and all studies were elevated. The ESR was 68mm (normal lab value <20mm), CRP was 12 (normal lab value <8.0) and platelet levels were 420,000 per µL of blood (normal lab value <400,000).
 |
|
Fig. 6. Visual field of the same patient. Click image to enlarge. |
I strongly suspected GCA and contacted his internist. In collaboration, we initiated 60mg PO of prednisone per day, and I ordered a temporal artery ultrasound—which ultimately was interpreted as being consistent with GCA—while his internist arranged a rheumatology consult. The patient reported that he felt immediately better on the steroid and that his headache and diplopia disappeared almost as quickly. He ultimately suffered no vision loss.
Case 5
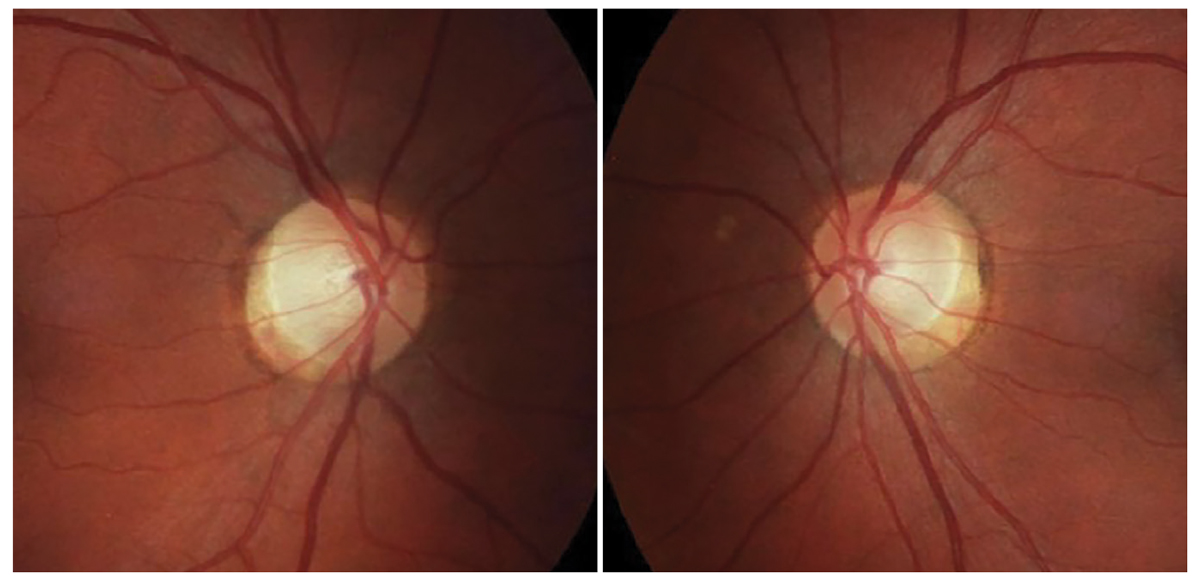
A 46-year-old woman was referred from a local optometrist for new-onset blurred vision in her right eye for the past month. She reported no pain or diplopia and felt that she might have had COVID one week before vision loss but did not test for the virus.
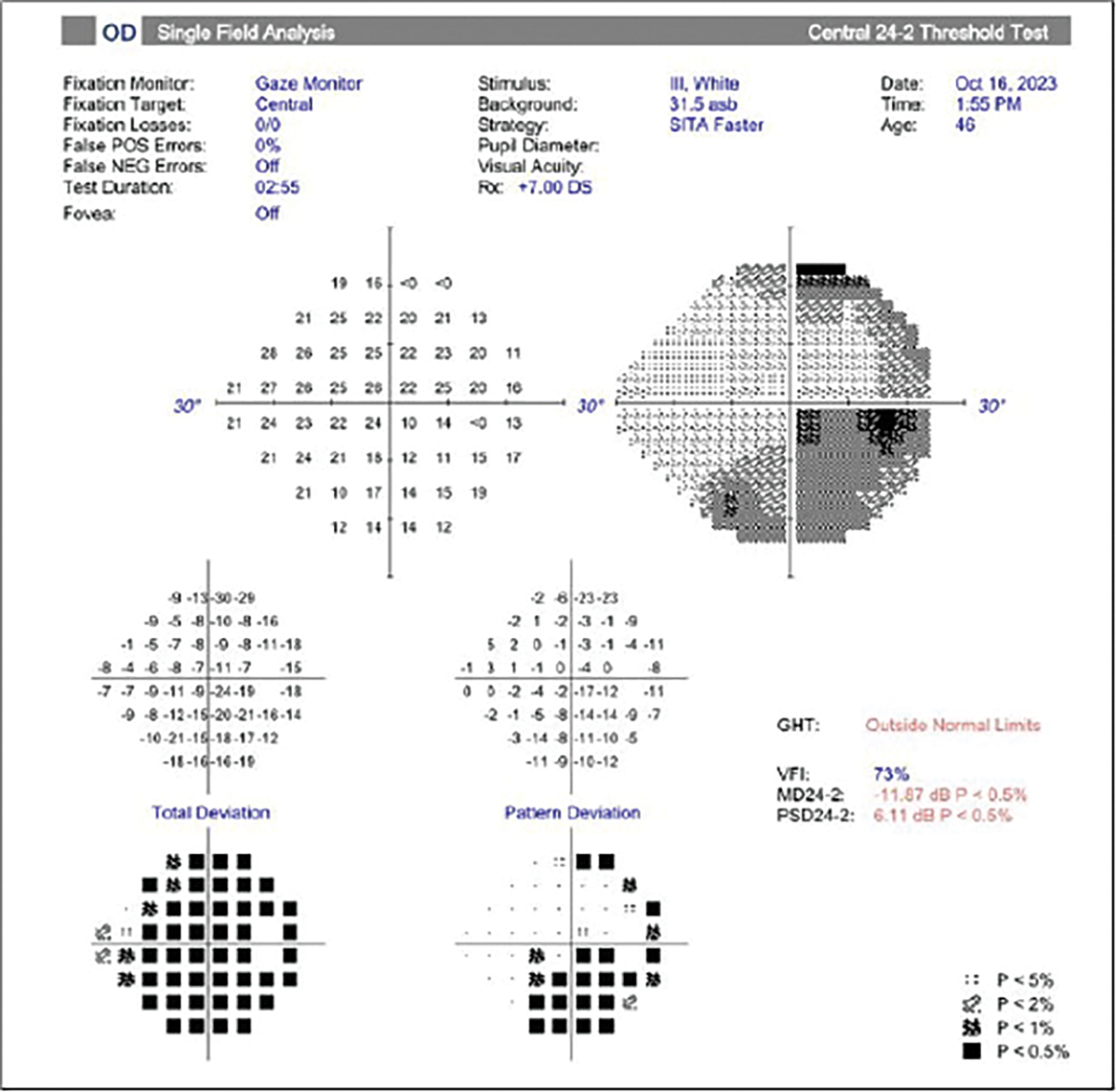
Her best-corrected VA was 20/60 OD and 20/20 OS with a RAPD OD. Her dilated exam revealed normal obliquely inserted optic discs without edema with cupping at 0.6/0.6 OD, OS (Figure 5). Her color plates were abnormal OD, with her identifying eight out of 14 plates OD and normal OS, identifying all of the plates correctly. OCT revealed a normal retinal nerve fiber layer (RNFL) and ganglion cell complex OU. Threshold perimetry was normal in her left eye, and there was an inferior defect in her right eye.
Though her vision loss was painless and consistent with non-arteritic ischemic optic neuropathy (NAION), her disc appearance did not correlate with this condition, which would be a small, crowded optic disc (Figure 6). Also, the lack of pain did not support a suspicion of retrobulbar optic neuritis. Upon further discussion, she acknowledged that she felt she was having trouble with her right contact lens for several months and thought that it was a refractive issue. She acknowledged that her vision loss may have been present for longer than the past month and that she may had just become aware of it.
In that she had an optic neuropathy, my first approach was to neuroimage her. At a commercial imaging center, I ordered an MRI of the brain, orbits and chiasm with and without contrast and with fat suppression for the orbital portion and attention to the sellar area. The tests came back revealing a planum sphenoidale meningioma compressing the prechiasmatic portion of the right optic nerve, abutting the chiasm and internal carotid artery. I discussed referral of the patient for the required surgical procedure with a local orbital surgeon and neurosurgeon who work in tandem with similar lesions, but they both declined the patient over significant risk due to the lesion’s proximity to the internal carotid artery and optic chiasm. They recommended a university skull-based surgeon.
 |
|
Fig. 7. Normal appearing optic nerve of the case 6 patient. Click image to enlarge. |
I explained the diagnosis to the patient and helped her get an appointment with the recommended surgeon. She ultimately underwent a craniotomy with tumor removal. Upon follow-up several months later, she felt her vision was better. Indeed, her acuity in the right eye was now 20/15, and her visual field defect disappeared. This recovery was expected due to her initial normal OCT findings that indicated her structural function was still normal, though her visual function had been impacted.
Patients presenting with compressive optic neuropathy are variably symptomatic, depending upon the duration, severity and etiology of the underlying condition. Painless VA decrease and visual field loss are the most common complaints.14-16 Another symptom may be decreased color perception. Occasionally, the patient may be asymptomatic and carry an erroneous diagnosis of amblyopia or glaucoma.14
Direct impingement of CN II is the mechanism of compromise in compressive optic neuropathy. Most often, this stems from a space-occupying mass within the orbit. Additionally, intracranial parasellar lesions such as pituitary adenoma, meningioma and craniopharyngioma can produce the same effect.
The underlying etiology must be identified to appropriately manage the condition. A directed laboratory analysis is usually prudent, particularly if thyroid disease is suspected. Imaging studies of the orbits and chiasm using contrast enhanced CT or MRI are critical in the diagnosis. In many cases, surgical treatment can significantly improve visual function, even in cases where there was poor initial VA.14-16
Meningiomas arising from the midline anterior cranial fossa may be classified as planum sphenoidale or olfactory groove meningiomas and account for 10% of intracranial meningiomas. These lesions may manifest with cognitive dysfunction or visual loss later as they grow. They may cause superior/posterior displacement of the frontal lobes and inferior/posterior compression of the optic chiasm, and most are treated with surgery.17,18
Case 6
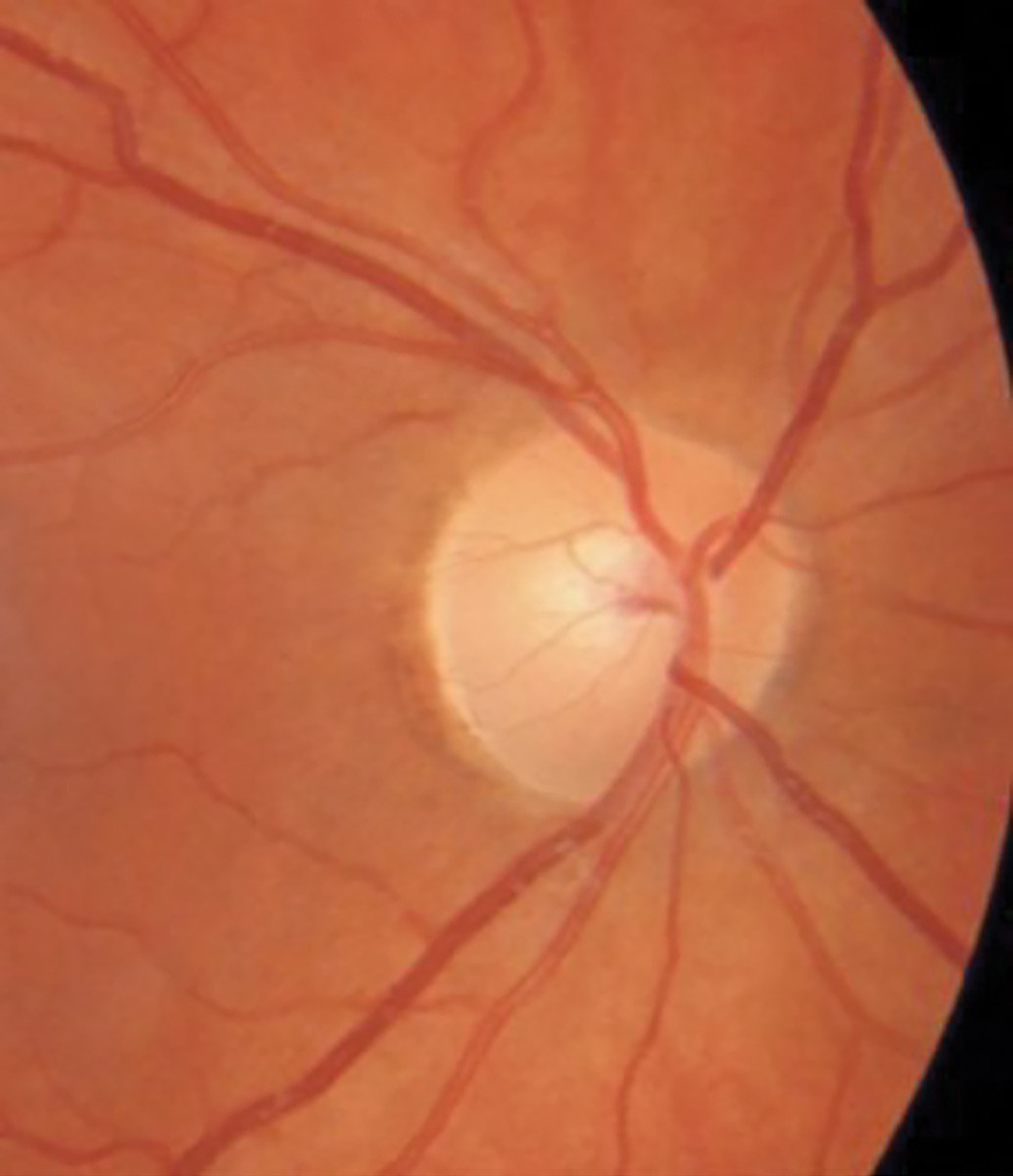
A 30-year-old woman was referred urgently from her internist for sudden painless vision loss in her right eye of several days’ duration. She had recently relocated to the area and had a previous medical history of an elevated antinuclear antibody count suggestive of systemic lupus erythematosus (SLE) that had never been fully evaluated or diagnosed and presumptive demyelinating disease of either multiple sclerosis or chronic inflammatory demyelinating polyneuropathy, which had also not been fully evaluated or treated. Prior to visiting her internist, she had presented to a local hospital ER with vision loss. According to the patient, she was told that her vision loss was either due to her lupus or multiple sclerosis and given erythromycin ointment for some ocular redness before being dismissed.
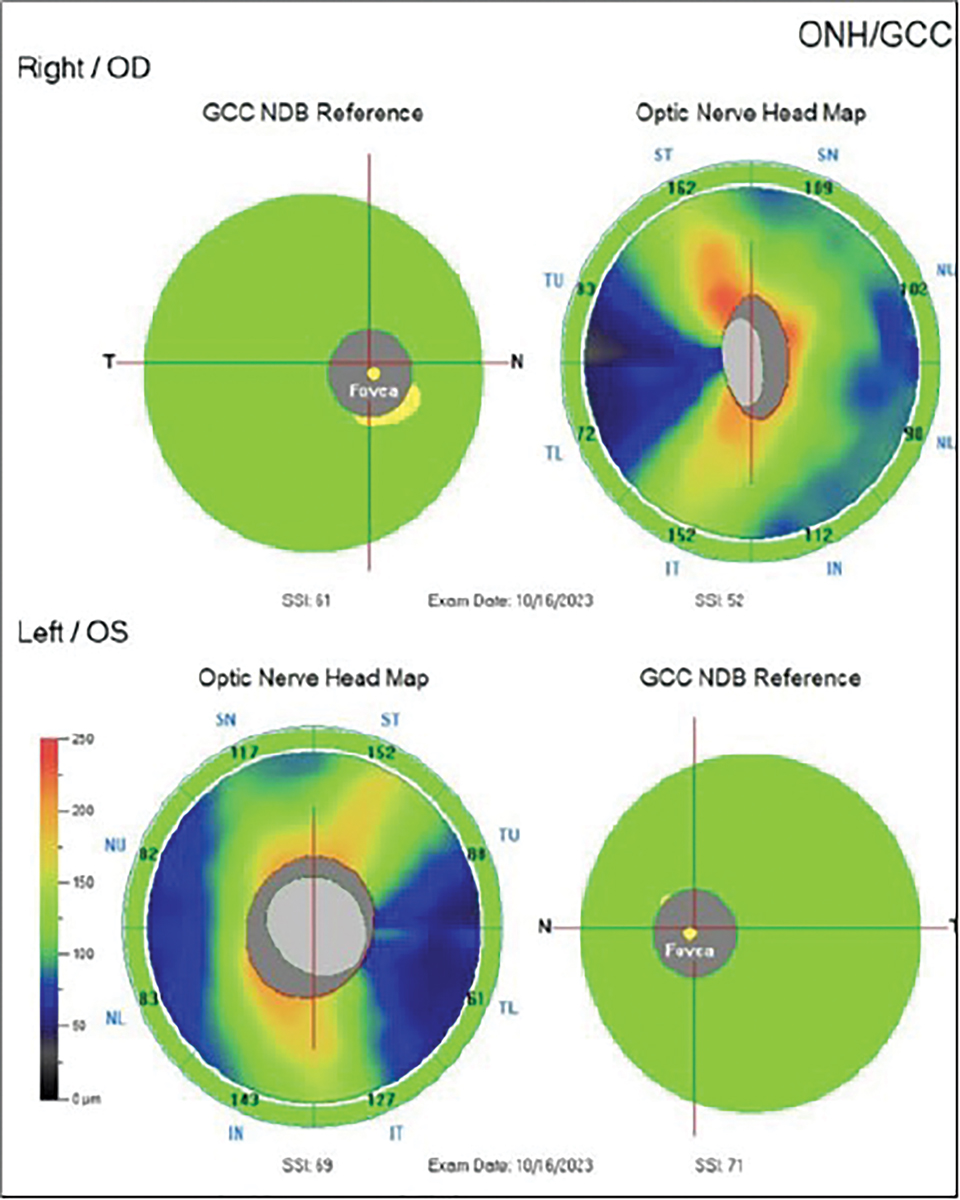
When I saw the patient, her VA was hand motion OD and 20/20 OS. Her pupils were equal and symmetrically responsive to light, and there was no RAPD noted in either eye. Her extraocular motility was full without restrictions, but curiously she reported diplopia despite her poor acuity in the right eye. Confrontation screening fields were full OS and could not be assessed in her right eye due to non-responsiveness. Threshold perimetry was not attempted as the patient felt that she could not perform the test with her poor vision. Upon examination, she had normal optic discs, and her OCT findings demonstrated a normal and symmetrical RNFL and ganglion cell complex in each eye (Figures 7 and 8).
 |
|
Fig. 8. This same patient’s normal OCT results. Click image to enlarge. |
Based upon her normal optic nerve appearance and questionable medical history of SLE and multiple sclerosis, a retrobulbar neuritis was considered. However, normal structure on OCT, lack of pain and absence of an RAPD in the involved eye made me suspicious. I ordered contrast-enhanced MRI of her brain, orbits and chiasm as well as antibodies to test for neuromyelitis optica spectrum disorder and myelin oligodendrocyte antibody disease, which should be considered in every evaluation for possible demyelinating optic nerve disease. I explained the urgency for testing and scheduled her for a one-week follow-up.
Results of neuroimaging came back promptly and showed several old demyelinating plaques suggestive of multiple sclerosis, but there were no acute lesions and no abnormal enhancement of the optic nerves or chiasm.
She missed her one-week follow-up appointment, and she was contacted and rescheduled the following week. She cancelled the subsequent appointment the day of and did not reschedule. Multiple calls to reschedule went unheeded. No antibody testing came back, presumably as it was unlikely done. To date, she has never reconnected to learn of her MRI results or follow-up on her reportedly marked vision loss. Based upon my initial suspicions from clinical findings and patient behavior, I have ascribed the incident to a case of non-organic vision loss. An important rule to remember is that a patient can misrepresent vision loss, but they cannot misrepresent a normal pupil response or normal RNFL.
Takeaways
There are numerous neuro-ophthalmic disorders that can afflict patients, with some being more common than others. Optometrists are positioned to assist in the diagnosis and management of these patients due to their understanding of the visual system. Participating in the care of patients with neuro-ophthalmic disorders requires a familiarity and comfort with ordering neuroimaging and blood work and developing a support network of other physicians including internists, rheumatologists, neurologists, neuro-ophthalmologists, vascular surgeons and neurosurgeons, to name a few. Armed with this experience, ODs can positively impact the clinical care outcome of patients with neuro-ophthalmic disease.
Dr. Sowka is a provider at US Eye/Center for Sight in Venice, FL where he focuses on glaucoma management and neuro-ophthalmic diseases. He is on the speaker bureau for Bausch Health.
1. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27(3):257-68. 2. Yanovitch T, Buckley E. Diagnosis and management of third nerve palsy. Curr Opin Ophthalmol. 2007;18(5):373-8. 3. Wilker SC, Rucker JC, Newman NJ, et al. Pain in ischaemic ocular motor cranial nerve palsies. Br J Ophthalmol. 2009;93(12):1657-9. 4. Zheng J, Wan Y. Internal carotid artery aneurysm with incomplete isolated oculomotor nerve palsy: a case report. J Med Case Rep. 2023;17(1):77. 5. Kung NH, Van Stavern GP. Isolated ocular motor nerve palsies. Semin Neurol. 2015;35(5):539-48. 6. Zhang X, Wei S. Etiology, localization of the lesion and prognosis for patients firstly diagnosed in ophthalmology department with oculomotor nerve palsy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020;45(12):1425-30. 7. Akagi T, Miyamoto K, Kashii S, et al. Cause and prognosis of neurologically isolated third, fourth or sixth cranial nerve dysfunction in cases of oculomotor palsy. Jpn J Ophthalmol. 2008;52(1):32-5. 8. Witthayaweerasak J, Tansuebchueasai N, Aui-Aree N. Clinical prediction score for early neuroimaging in acquired isolated oculomotor nerve palsy. Eye Brain. 2020;12:89-95. 9. Bajpai V, Madan S, Beri S. Arteritic anterior ischaemic optic neuropathy: an update. Eur J Ophthalmol. 2021;31(6):2818-27. 10. Nordberg C, Johansson H, Petursdottir V, et al. The epidemiology of biopsy-proven giant cell arteritis: special reference to changes in the age of the population. Rheumatology. 2003;42(4):549-52. 11. Ness T, Nölle B. giant cell arteritis. Klin Monbl Augenheilkd. 2024;241(5):644-52. 12. Hansen MS, Terslev L, Jensen MR, et al. Comparison of temporal artery ultrasound vs. biopsy in the diagnosis of giant cell arteritis. Eye (Lond). 2023;37(2):344-9. 13. Chan CC, Paine M, O’Day J. Steroid management in giant cell arteritis. Br J Ophthalmol. 2001;85(9):1061-4. 14. Laowanapiban P, Sathianvichitr K, Chirapapaisan N. Structural and functional differentiation between compressive and glaucomatous optic neuropathy. Sci Rep. 2022;12(1):6795. 15. Jiang L, Shi J, Liu W, et al. [Clinical feature of chronic compressive optic neuropathy without optic atrophy]. Zhonghua Yan Ke Za Zhi. 2014;50(12):889-93. 16. Sun CB, Jiang B, Liu GH, Xiao Q. [Clinical and imaging characteristics of optic nerve tumors as the differential diagnosis of optic neuritis]. Zhonghua Yan Ke Za Zhi. 2023;59(5):367-75. 17. Rath S, Varma DR, Tibrewal S, et al. A flower in the brain: planum sphenoidale meningioma. Indian J Ophthalmol. 2020;68(11):2514-16. 18. DeMonte F, Raza SM. Olfactory groove and planum meningiomas. Handb Clin Neurol. 2020;170:3-12. |

