A patient presenting with diplopia—whether horizontal, vertical or diagonal—is often a clinical challenge.1 Constant diplopia with acute onset will have different differentials than intermittent diplopia, for example.2,3 While the cause can be benign, some cases, such as those accompanied by new headache, ocular pain, unilateral pupil dilation, muscle weakness, ptosis, trauma or papilledema, raise red flags for immediate referral.4,5 Most etiologies will fall into one of five categories: (1) refractive, (2) binocular vision disorder, (3) orbital disease, (4) neuromuscular junction dysfunction, or (5) injury to the central nervous system/cranial nerves (CNs).6 A systematic approach to the differentials is key to identifying and treating benign causes—and promptly referring patients when it is vision or life threatening.
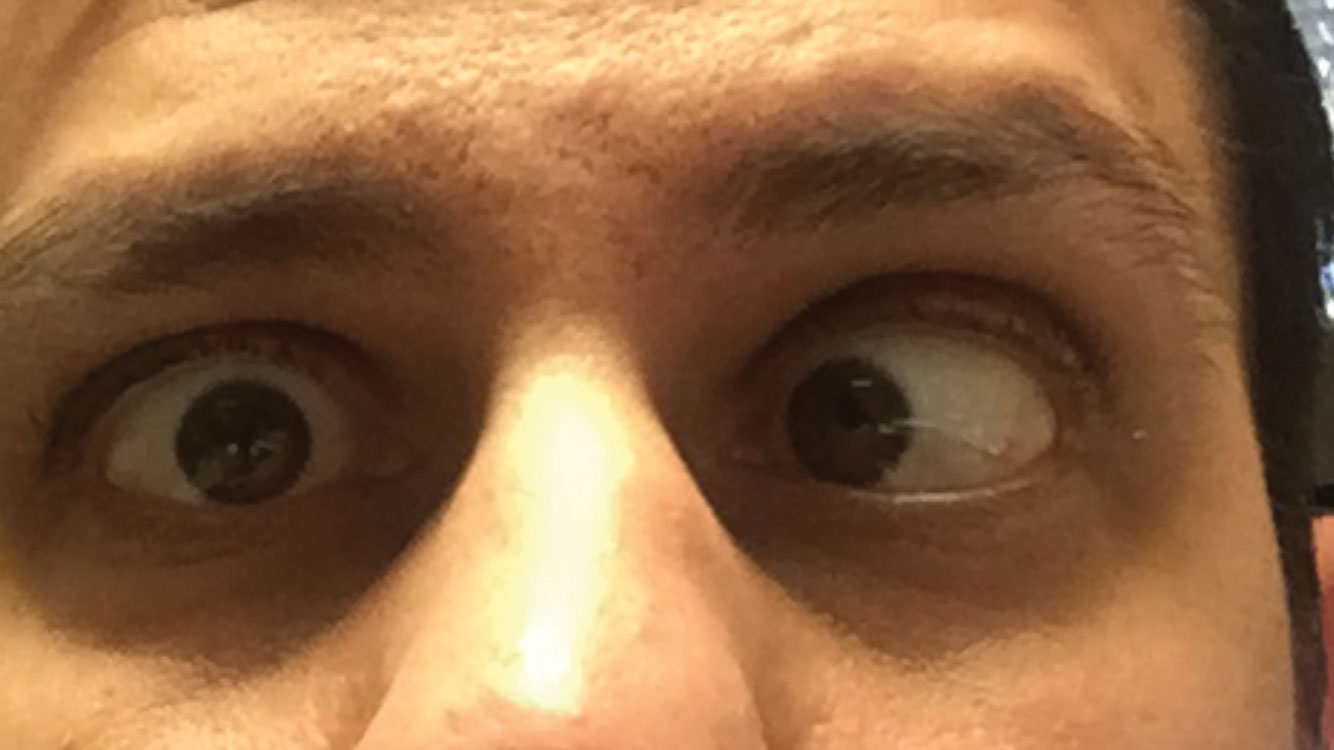 |
| CN VI palsy, seen here in the right eye, accounts for 50% of all isolated CN palsies. |
Patient History
The first step on the path to proper identification is a thorough patient history. The clinician must determine if the diplopia is monocular or binocular, as binocular diplopia may have a life-threatening cause.3,4
Monocular Diplopia
Diplopia that persists when one eye is covered falls into the category of monocular diplopia, or polyopia (greater than two images). Clinicians should have the patient cover each eye separately when testing for monocular diplopia. This finding is rarely due to cortex lesion and is generally attributable to causes within the eye itself. Decreased vision due to uncorrected astigmatism, dry eye and tear film deficiencies, corneal pathology or scarring, iris abnormalities, lenticular changes, vitreal opacities and macular disease are all possible causes of monocular diplopia.4,7,8 Medications (e.g., antidepressants, antihistamines, diuretics) may contribute to ocular surface dryness and induce a monocular diplopia.9
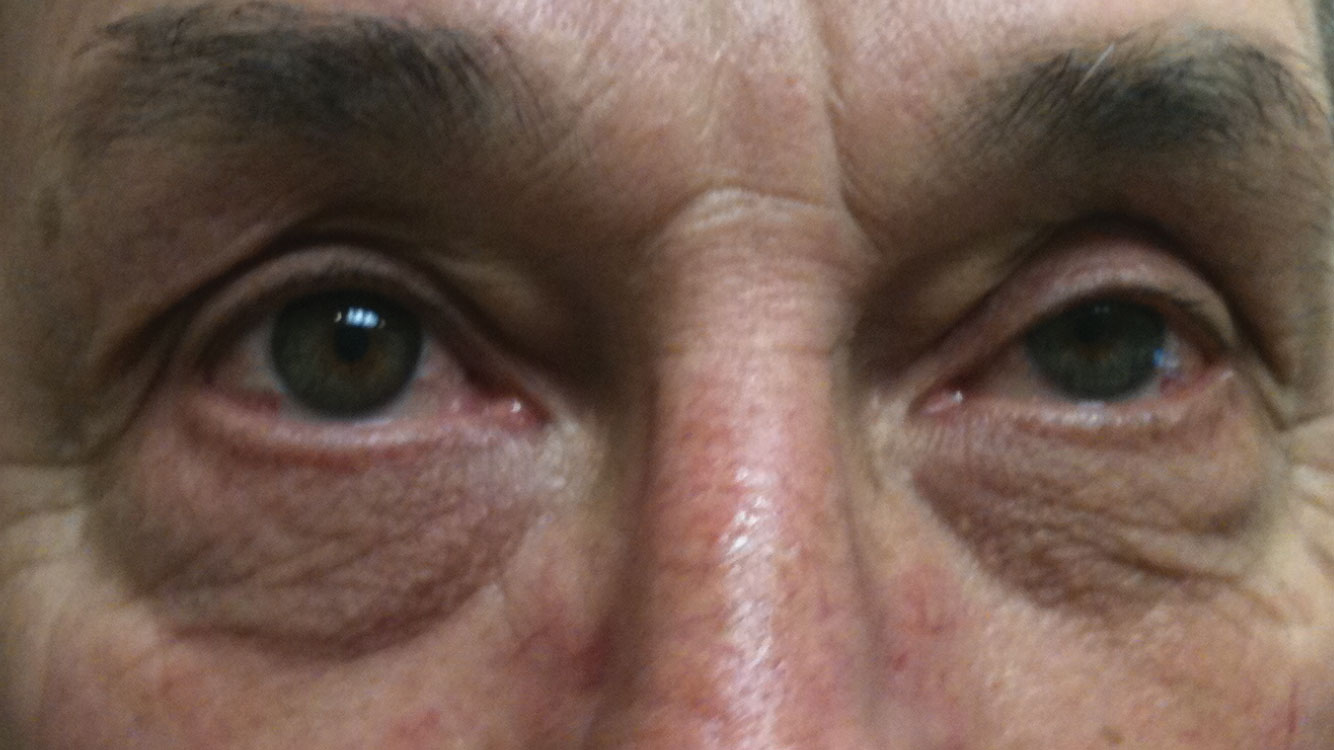 |
| As many as 60% of MG patients, such as this one, present with ptosis and diplopia. |
Binocular Diplopia
Unlike monocular diplopia, binocular diplopia, due to ocular misalignment, will disappear when either eye is covered. The type of diplopia the patient complains of—horizontal, vertical or diagonal; worse at distance or near; increased or decreased in a particular gaze position—helps to identify which extraocular muscle is involved. A thorough systemic health history and step-by-step examination is key to localizing most underlying etiologies.2,3,10 A systemic health history should include questions regarding trauma, diabetes, hypertension, thyroid disease, cancer, infection and immunosuppression—all of which could cause CN palsies and diplopia through vascular or restrictive mechanisms.2,6,10
Although less frequently, certain medications can cause binocular diplopia, such as anticonvulsants, selective serotonin reuptake inhibitor antidepressants, erectile dysfunction medications, migraine therapies and other medications with anticholinergic properties. Many antidepressants may aggravate the symptoms of a convergence insufficiency by affecting accommodation.6,11
Ocular motility and alignment testing may include the cover/uncover test, alternate cover test, Maddox rod and corneal light reflex. Ocular misalignment may be caused by a tropia, and an obvious eye turn is noted. A phoria occurs when the misalignment is not obvious, and diplopia occurs only when binocularity is disrupted. A key point in alignment testing is the evaluation for comitancy, in which the size of an ocular deviation remains the same in all directions of gaze. A comitant deviation, such as a decompensating heterophoria, presents with an intermittent or gradual onset, shows full range of ocular movement in all positions of gaze and may have a history of childhood strabismus.12 In contrast, CN palsies and extraocular muscle restrictions cause non-comitant deviations with the greatest diplopia noted in the direction of action of the weakened muscle.2,3,9,10 Clinicians must examine each eye separately (ductions) to catch a subtle restriction that could be missed when evaluating both eyes together.3,5,13 To test versions, the patient fixates on a target that is slowly moving laterally while the clinician checks the medial rectus of the adducting eye and the lateral rectus of the abducting eye. The target is then moved superiorly to evaluate the superior/inferior rectus of the abducted eye and the inferior/superior oblique of the adducting eye. The test is repeated on the opposite side to test contralateral gaze.3,5,13 Forced duction testing can identify muscle restriction such as in thyroid disease or muscle entrapment by a fracture following trauma.3,5,13
Horizontal diplopia, when the images are truly side-by-side, is suggestive of a medial or lateral rectus under action or restriction.2,3,9,10 Horizontal diplopia present only at near, and especially noted with prolonged near work, is more likely attributable to a convergence insufficiency, which can occur in children and adults idiopathically. Convergence insufficiency can occur after trauma, in neurodegenerative diseases such as Parkinson’s disease and with medications that have an anti-cholinergic effect on accommodation.7 Differential diagnosis for horizontal diplopia at distance includes unilateral or bilateral CN VI palsy, internuclear ophthalmoplegia (INO), age-related decompensating esophoria or muscle restriction, most commonly from thyroid disease, a space occupying lesion or myasthenia gravis (MG).2,9,10
Vertical diplopia assessment involves the four remaining muscles: the superior and inferior recti and the superior and inferior oblique. CN III and IV palsies, skew deviations (with or without INO), muscle restrictions and decompensated phorias can all cause vertical diplopia.9
Some diseases may cause variable patterns of horizontal, vertical and oblique diplopia throughout the day. Diplopia that varies throughout the day, improves with rest and may have an associated ptosis is highly suggestive of MG as the underlying cause.7 Other differentials to consider that present with a variable pattern of diplopia include thyroid eye disease, Guillain-Barré syndrome, Parinaud syndrome, Miller-Fisher syndrome, trauma, Parinaud (dorsal midbrain) syndrome and Wernicke’s encephalopathy.2,9,14
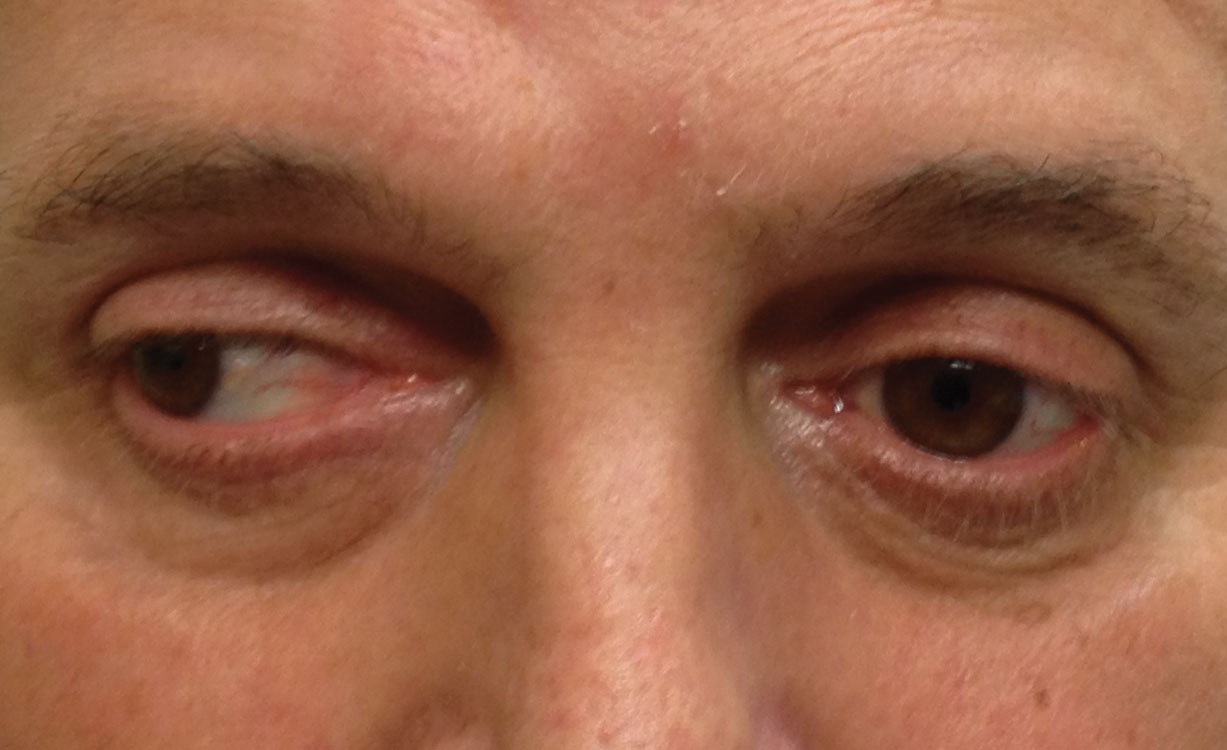 |
| This patient has longstanding medial rectus palsy secondary to facial trauma. |
Here some common underlying etiologies of binocular diplopia:
Refractive. Misalignment of the optical centers of prescription glasses or non-prescription reading glasses, poor fitting glasses and the edges of high prescription glasses may all cause diplopia or worsen an existing heterophoria. Aniseikonia from refractive error results in differences in image size and shape in the visual cortex, causing diplopia when wearing glasses. Contact lens use often resolves the image difference in most cases of aniseikonia.1
Binocular vision disorder. A patient with a history of childhood strabismus may develop diplopia later in life due to a decompensation of their misalignment.10 Decompensating phorias and vergence problems are the most common cause of diplopia at near only. Asthenopia occurs with extended near activities, resulting in diplopia and headaches. Convergence insufficiency results in diplopia after prolonged near work and may be associated with uncorrected refractive error, dry eye and Parkinson’s disease.1
Orbital disease. Thyroid eye disease (TED), idiopathic orbital inflammation and orbital tumors are the most common extraocular muscle and orbital diseases that cause diplopia.2,3 Orbital inflammation is usually unilateral and may affect the orbital fat, extraocular muscles, lacrimal gland, sclera or optic nerve. Onset may be sudden and painful, and the eye may appear proptotic. This has been associated with rheumatoid arthritis, sarcoidosis and, less frequently, giant cell arteritis (GCA). Testing includes rheumatoid factor, chest x-ray and ACE level for sarcoidosis and anti-nuclear antibody for systemic lupus erythematosus.
TED predominantly occurs in hyper-thyroid states, although approximately 10% of patients can present with hypo- and euthyroid states, which may not correlate with the thyroid status.8 Lab testing includes thyroid function and thyroid antibody tests, and risk factors are higher in females, smokers and those with family history of disease.3 Painless proptosis, muscle restriction, lid retraction and variable lymphocytic inflammatory infiltration are notable findings that occur in approximately 50% of patients with Graves’ Disease.3 The inferior and medial rectus are the most affected, causing a vertical diplopia due to restriction in elevation and an esotropia due to restricted abduction.2,3,5,8,9 Most patients have a mild form of TED, but 3% to 7% will have vision-threatening concerns from corneal disease or optic nerve compression.8 A CT scan of the orbit will assess the extraocular muscles and optic nerve and reveal muscle enlargement and risk of optic nerve impingement.8
Other, less common, orbital causes of diplopia include trauma and neoplasms. A blow-out fracture is an emergent situation, as the sinus can cause a negative pressure that pulls on the inferior orbital wall, trapping the inferior rectus muscle, resulting in an inability to elevate the affected eye and vertical diplopia.3,6
The orbital floor in adolescents is flexible and can quickly open and close, trapping the inferior rectus, and may present with no other obvious signs of trauma. Patients with a history of orbital trauma and a white eye (or lack of subconjunctival hemorrhage) will need emergent imaging to determine if there is entrapment of the inferior rectus, especially in children. In these cases, decompression surgery is urgently needed within 24 to 48 hours to avoid ischemia of the muscle.15 Conversely, if there is no muscle entrapment, due to orbital floor fracture, surgical intervention may be considered in two weeks.16
Neoplasms and sinus-related issues should be considered in the presence of a correlating health history. Secondary orbital tumors, lymphomas and metastatic cancers are the most common orbital neoplasms presenting with unilateral proptosis and resistance to retropulsion.3 All patients with a new onset of diplopia and a history of cancer should have urgent imaging studies.17 Rarely, a silent sinus syndrome will cause a downward displacement and enophthalmos of the eye. The obstruction of the ostium of the maxillary causes a negative pressure that pulls downward on the inferior orbital wall, resulting in a vertical diplopia.3,6,18
Giant cell arteritis. A patient with GCA can present with any CN palsy.10 GCA should be ruled out in all patients who present with diplopia, especially those older than age 60. Urgent blood work should include complete blood count, c-reactive protein (CRP) and sedimentation rate (ESR). If the platelet count, CRP and ESR are elevated or GCA is suspected based on accompanying scalp tenderness, headache, fever and generalized malaise, clinicians should refer the patient for urgent treatment.8,19 Studies indicate that GCA is the underlying cause of diplopia in anywhere from 3% to 15% of presenting cases of diplopia with biopsy proven GCA, but the risk of morbidity and mortality is too high to miss this disease.8,19
Neuromuscular junction dysfunction. MG is the classic neuromuscular junction disease that can become life threatening when it affects the muscles of respiration, causing respiratory failure. Approximately 50% to 60% of MG patients present with a ptosis and diplopia, and approximately 20% to 30% have localized ocular involvement.5,8 The most common age of onset is in the third decade for women and the seventh decade for men.5 Weakness of the medial rectus is fairly common, but diplopia can vary between horizontal, vertical and oblique. Patients report variable fatigue and ptosis of one or both eyelids that worsens with prolonged activity or toward the end of the day. However, MG can cause a fluctuating diplopia at any time of the day, even on waking.9 A recent history of weakness and difficulty walking or swallowing are found in generalized MG but absent in the ocular form. As with TED, clinicians should remain suspicious of MG in all cases, as it can mimic CN IV, VI and partial CN III palsies in addition to INO, although the pupil is never involved.2,3
Several in-office tests are available to help support the diagnosis of MG. During the Cogan lid twitch test, the patient looks down for a few seconds and the clinician then watches the lid reaction when they return to primary gaze. A 1mm to 2mm drop of eyelid elevation immediately after returning to primary gaze is a positive response. Application of ice packs for one to two minutes or a resting state for 10 minutes is an another easy in-office test, and an improvement in the ptotic eyelid is a positive response in suspected cases of MG. Fatigue in prolonged upgaze for at least two minutes with a resulting ptosis, worsened ptosis or inability to maintain upgaze is considered a positive test.2 Approximately 15% of MG patients will have thyroid changes and co-existing TED, while about 10% will have thymoma present and will be evaluated for surgery.5
Internuclear ophthalmoplegia. This is a lesion or injury of the medial longitudinal fasciculus. Clinically, the patient will not be able to adduct the affected eye (or look nasally) and the non-affected eye will show an abducting nystagmus (when looking temporally); convergence, if present, will be spared.3 An INO may occur unilaterally or bilaterally, and a review of 410 inpatients in 33 years shows the underlying cause can be divided into three major categories: (1) stroke, (2) multiple sclerosis (MS) and (3) other causes such as trauma, injury, infection, tentorial herniation, tumor and GCA.14 A lesion in the pontine or para-pontine area can cause a gaze palsy opposite the INO, resulting in a “one-and-a-half syndrome.” An INO that presents bilaterally results in a large exotropia in both eyes causing a “wall-eyed effect.”9 In patients with MS, an INO is the most common motility abnormality and is present in as many as 53% at some point in their illness.8 Patients presenting with an INO should be urgently sent for imaging and bloodwork.
Cranial nerve palsies. CN III, IV and VI palsies share many of the same underlying etiologies such as microvascular CN palsies, intracranial aneuryms and neoplasms.10,20 Trauma can impair the function of any nerve, but CN IV in particular is more susceptible to trauma. Microvascular disease accounts for many CN palsies in patients older than 50, especially in those with known microvascular disease. Pain and rapidity of onset provide less definitive clues about cause, should a cerebrovascular accident be suspected. Pain can be severe or absent in aneurysmal CN III palsies and ischemic events, though a significant headache and CN III palsy requires careful pupil testing and referral to an emergency room. Research suggests acute onset is associated with ischemic events while slow onset is associated with compressive cases.20 A CN VI palsy is the most common, followed by CN IV and CN III.7 In all cases of nerve palsies, evaluation must carefully determine if single or multiple nerves are involved, as imaging is most often warranted, particularly when multiple cranial nerves are involved.21
CN VI innervates only the lateral rectus muscle, and paralysis causes an estropia from an abduction deficit. It is the most common isolated ocular motor palsy and accounts for 50% of them.8 The patient reports horizontal diplopia that is worse at distance and worse when looking in the direction of the affected muscle. Microangiopathic disease causes up to 36% of isolated, acute CN VI palsies in patients older than age 50 with vascular risk, and diplopia spontaneously resolves within two to three months.2,8 Wernicke’s encephalopathy, MS and Duane’s retraction syndrome may be mistaken for a CN VI palsy and should be considered in the differentials.2,5,7 In all other patients with an acute CN VI palsy, assessment for causes such as GCA, tumors, intracranial hemorrhage and trauma warrant referral to the emergency room for evaluation and imaging studies.8,17 If increased intracranial pressure is the underlying cause of a CN VI palsy, a thorough evaluation should include optic nerve head assessment for the presence of associated papilledema.8,17
A CN IV palsy affects the function of the superior oblique muscle, resulting in a vertical oblique diplopia more noticeable in downgaze.9 Trauma in the most common due to the long course of the nerve around the midbrain.5 In the absence of trauma, clinicians should test to rule out TED and MG.7 As with CN VI palsies, microangiopathy is the major cause of a CN IV palsy in patients older than 50.7,8 The pneumonic GOTS—gaze opposite, tilt same—indicates there is a greater vertical deviation when the patient looks to the opposite side of the affected muscle or tilts their head to the same side. For example, a right superior oblique impairment will have a right hypertropia: greater diplopia with a right head tilt and when looking to the left. The patient will have a left head tilt to minimize their diplopia.5,7
A decompensated congenital CN IV can be distinguished from an acute CN palsy by evaluating vertical fusional amplitudes with prism bars or the amount of ocular rotation between the eyes along with the size of the vertical deviation.10 The prism bar test is performed by measuring the range of prism that will eliminate the diplopia. Normal vertical fusional amplitudes range from one to four prism diopters, whereas patients with congenital strabismus may demonstrate up to six prism diopters of vertical fusion amplitude.10,20 A review of old photos may also help to identify those patients with longstanding congenital palsies.
CN III innervates the inferior oblique and the superior, inferior and medial recti muscles. A complete oculomotor palsy results in complete ptosis, a mid-dilated pupil and an eye that appears “down and out.” Patients report an oblique diplopia when the eyelid is lifted. Any CN III palsy needs immediate imaging, including CTA or MRA, as compression from an aneurysm of the posterior communicating artery is the most common etiology of a complete palsy with pupil involvement and is life threatening. Ischemic or microvascular causes are more common, and the diplopia often improves during the recovery from the event. The presence of pain may occur in both scenarios but does not help to differentiate between them.
However, most CN III palsies are not complete, and clinicians must use cover testing to catch a subtle signs.
Patients with palsies with ischemic causes are usually older with risk factors such as diabetes, hypertension, hyperlipidemia and tobacco use. Pupil evaluation may help narrow the differential, as the pupillary fibers reside on the dorsomedial aspect of the oculomotor nerve and are affected in 90% of compressive pathologies, causing a fixed, dilated pupil. In contrast, microvascular ischemia causes an infarct in the center of the nerve, which spares the pupil in 70% of ischemic cases. Up to 30% of ischemic palsies will have an anisocoria of 1mm to 2mm. Ischemic palsies usually improve within three months, and never demonstrate aberrant regeneration. The pupil rule cannot be applied to rule out a compression lesion when the palsy in incomplete.2,5,9,20
In addition, all patients with a new onset diplopia and a history of cancer require urgent imaging studies to rule out a metastatic lesion.17
Researchers have debated the use of imaging in all CN palsies for some time, and most agree those with an acute isolated CN III palsy need urgent imaging to rule out a compressive aneurysm or suspected cavernous sinus thrombosis. Imaging of the brain and orbits is appropriate in suspected retro-bulbar mass, TED or orbital trauma. In patients older than 60, referral for urgent bloodwork is indicated to rule out GCA.8
However, a literature review shows that, for CN VI palsies, no definitive answer for imaging exists, as both prospective and retrospective cohorts had valid arguments for their conclusions of imaging all patients with isolated CN VI palsies. Thus, clinicians should always consider imaging CN palsies, especially when presenting with other neurological signs and symptoms.21
Diplopia can be a concerning condition for any clinician to address. The key to following the right course of action is determining the underlying etiology. Primary care optometrists often have patients complain of diplopia, and with the right tools and skills, every OD can properly treat, coordinate a proper referral and often reassure the patient with a benign presentation.
Dr. Suhr is chief of the Optometry Section at the Philadelphia Corporal Michael J. Crescenz VA Medical Center.
Drs. Chubb and Himmelein are staff optometrists at the Philadelphia Corporal Michael J. Crescenz VA Medical Center.
1. Mashige KP, Munsamy AJ. Diplopia. South African Family Practice. 2015;58(sup1):S12-17. 2. Rucker JC, Tomsak RL. Binocular diplopia. The Neurologist. 2005;11(2):98-110. 3. Dinkin M. Diagnostic approach to diplopia. Continuum (Minneap Minn). 2014;20:942-65. 4. Low L, Shah W, MacEwen CJ. Double vision. BMJ. 2015;351:h15385. 5. Aminoff MJ, Josephson SA, eds. Neuro-Ophthalmology in Medicine, 5th ed. Philadelphia: Elsevier Science; 2014:487-99. 6. Alves M, Miranda A, Narciso MR, et al. Diplopia: a diagnostic challenge with common and rare etiologies. Am J Case Reports. 2015;16:220-23. 7. Iliescu DA, Timaru CM, Alexe N, et al. Management of diplopia. Romanian J Ophthalmol. 2017;61(3):166-70. 8. Margolin E, Lam CTY. Approach to a patient with diplopia in the emergency department. J Emerg Med. 218;54(6):799-806. 9. Friedman D. Pearls: diplopia. Sem Neurol. 2010;30(1):54-65. 10. Peragallo J, Newman N. Diplopia—an update. Sem Neurol. 2016;36(4):357-61. 11. Alao A, Lewkowicz C. Seeing double: sertraline and diplopia: a case report. Internat J Psychiatry in Med. 2015;49(1):107-10. 12. O’Colmain U, Gilmour C, MacEwen CJ. Acute-onset diplopia. Acta Ophthalmologica. 2013;92(4):382-86. 13. Pelak VS. Evaluation of diplopia: an anatomic and systematic approach. Hospital Physician. 2004;40(3):16-25. 14. Keane JR. Internuclear ophthalmoplegia. Arch Neurol. 2005;62(5):714. 15. Hammond D, Grew N, Khan Z. The white-eyed blowout fracture in the child: beware of distractions. J Surg Case Rep. 2013;(7):rjt054. 16. Grob S, Yonkers M, Tao J. Orbital fracture repair. Seminars in Plastic Surg. 2017;31(1):31-9. 17. Kirsch CFE, Black K. Diplopia: what to double check in radiographic imaging of double vision. Radiologic Clinics of North America. 2017;55(1):69-81. 18. Saffra N, Rakhamimov A, Saint-Louis LA, et al. Acute diplopia as the presenting sign of silent sinus syndrome. Ophthalmic Plast Reconstr Surg. 2013;29(5):e130-1. 19. Gupta PK, Bhatti MT, Rucker JC. A sweet case of bilateral sixth nerve palsies. Surv Ophthalmol. 2009;54(2):305-10. 20. Cornblath WT. Diplopia due to ocular motor cranial neuropathies. Continuum (Minneap Minn). 2014;20(4 Neuro):966-80. 21. Murchison AP. Neuroimaging and acute ocular motor mononeuropathies. Arch Ophthalmol. 2011;129(3):301-5. 22. Elder C, Hainline C, Galetta SL, et al. Isolated abducens nerve palsy: update on evaluation and diagnosis. Curr Neurol Neurosci Rep. 2016;16(8):69. |

