When patients in their early to mid-forties remark, “Doc, I used to always have great vision, but now I feel as though I can’t see anything without my glasses,” any optometrist knows they’re likely dealing with presbyopia. This pervasive condition affects roughly a quarter of the entire global population.1 Unfortunately, 826 million are likely to have limited daily function because they don’t have adequate management or correction.1
Optometrists can make the difference and offer patients corrective lenses and other solutions.2,3 But that’s not the end of their problems. Presbyopia can complicate, and be complicated by, a number of other conditions. Optometric physicians will need to consider the impact of their patient’s visual condition when fighting conditions such as dry eye, cataracts, retinal disease or prior refractive surgery. This article helps ODs navigate the necessary considerations when managing presbyopia in complex situations.
 |
The trial frame is a valuable clinical tool that allows our patients to see what we intend on prescribing, and feel how the prescription acts on their visual system. Prism and astigmatism can cause disruption to the vergence system and should be tried at all ranges before prescribing. This is an underused technique that all optometrists should rely on for most refractive and prismatic exams. Here, the patient is holding a near card with her prescription in the trial frame at the desired focal point. She is new to reading glasses and was given the “wow” factor before ordering her glasses. Click image to enlarge. |
Prism and Progressive Lenses
Purposeful prism can certainly be introduced into progressive lenses for presbyopic patients with diplopia, strabismus and other binocular vision conditions.2,4 Success with prism requires a delicate balance of objective and subjective tolerances; thus, doctors must tailor parameters for prescribing to the individual patient, taking into account their age, systemic and ocular histories, and common patterns of visual demands.2-4
While cover test and prismatic implementation into a trial frame can be rudimentary, there are some pearls to keep in mind when fitting progressive lenses. Prism appears in spectacles whenever the thickness of the lens varies between two points.5,6 Keep this in mind as we move into progressive lenses that combine multiple powers across one lens surface.6,7 As we have learned with Prentice’s rule, the prismatic effect on any point of a lens is directly proportional to the power of the lens, and the distance of that point from the optical center.5-7 Measuring the interpupillary distance is very important in progressive lenses and needs to be done with the head properly aligned. Remember to maintain normal head posture while performing cover testing as well as while measuring for spectacles.7 Any head tilts or turns can throw off the desired prismatic effect and may cause unwanted diplopia in itself.6,7
When considering the binocular prismatic consequence of spectacles, we focus on the net prismatic effect between the right and left lenses.5-7 This is known as the prismatic imbalance, which affects binocular fusion. For vertical prism, bases oriented in the same direction between the two eyes have canceling effects, while the opposite is true for horizontal prism.6,7 Make sure the amount of prism can be tolerated by the fusional vergence system by using a trial frame, along with the correct orientation of prism between the two eyes.6,7
Fresnel prisms can accommodate high ranges of prismatic correction.8 They consist of continuous thin, narrow prisms arranged on a plastic sheet.8 Because their design is dependent upon prismatic angle vs. thickness of the lens, they are thin, flexible, and discrete on the surface of spectacles.8 Fresnel prisms come in powers up to around 40 diopters.8 They are a good trial prism for patients using prism for the first time or in cases with a large change in prismatic correction. Fresnel prism, while very useful, does tend to degrade image quality and can be noticeable to the naked eye. Once the patient reports good success and comfort with stick-on Fresnel, prism can then be ground into lenses, a more permanent modality to prismatic correction.
Some diplopic patients with strabismic amblyopia may not achieve single vision with any amount of prism and may warrant patching to resolve symptoms.5,7,8 The same may be true for other types of diplopia secondary to neurologic concurrent pathologies causing progressive amblyopia over time. Some patients may also have some latent strabismus and may need multiple follow-up visits to ensure proper compensation and resolution in symptoms.
Adaptation to progressive lenses can take time and requires patience in addition to careful consideration. In a study, patients who couldn’t adapt to progressives demonstrated slower peak velocities in convergence responses, a weaker ability to modify convergence responses, a reduced rate and magnitude of phoria adaptation and a reduced vergence facility compared to successful wearers.4 These results suggest that when the accommodative system decreases in presbyopic subjects, the adaptive role of vergence and phoria systems may become critical when adapting to new visual environments such as those created when using progressive lenses.4 The ability to change convergence peak velocity had the greatest sensitivity and specificity compared to the other parameters.4
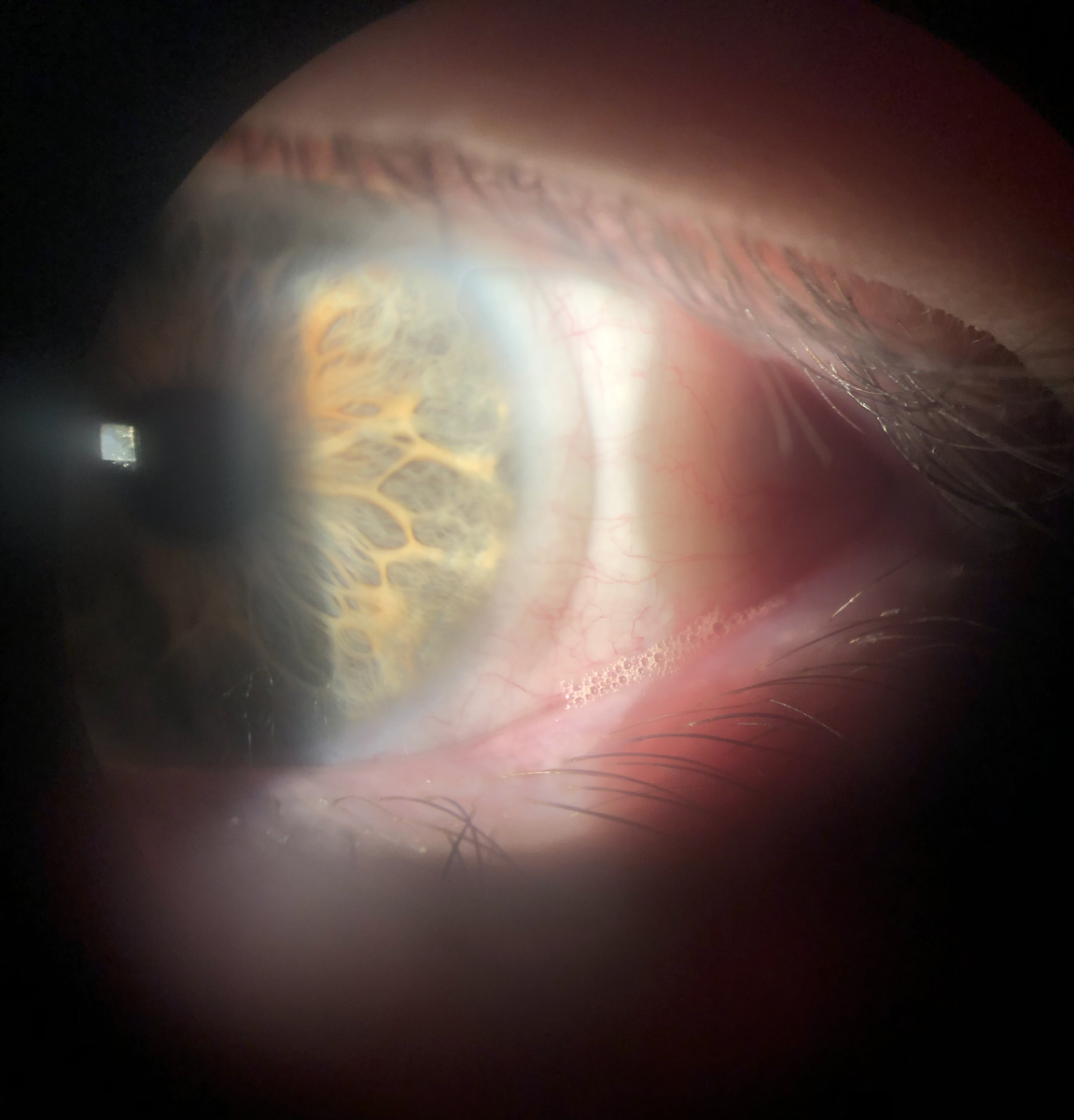 |
A bandage contact lens used on a keratoconjunctivitis sicca patient with significant epithelial staining, after which symptoms resolved and redness subsided. Note the frothing along the lower lid margin, indicative of meibomian gland dysfunction and reduced tear quality. Patients with ocular surface compromise often experience visual fluctuations that reduce the quality of multifocal contact lens wear. Click image to enlarge. |
The Ocular Surface
In all varieties of dry eye, especially in cases of severe ocular surface disease, presbyopia adds a lay\er of complexity to the already compromised patient. According to 2018 data from the US Bureau of Labor Statistics, 65% of the civilian labor force is age 35 or older, and this category is projected to maintain that average through 2028.9-11 This places eye care physicians in a pivotal position. Our aging patient population is learning to battle functional, progressive visual changes and adapting to these changes on top of the use of glasses can be difficult to navigate.
As stated by the epidemiology report from the Tear Film and Ocular Surface Society’s second Dry Eye Workshop report (DEWS II), for all subgroups analyzed the prevalence of dry eye increased significantly and showed a linear association with age.12 Research suggests that multiple factors, including uncorrected presbyopia, are associated with both ocular and nonocular symptoms.13 In fact, a 2017 study showed an increase in dry eye disease in patients who are presbyopic.14
Environmental factors such as pollen or dander allergies, prolonged digital screen time and contact lens wear can worsen dry eye signs and symptoms. In advanced stages of the condition, the severity of dry eye damage may become sight-threatening.15 And the medications that patients use—including antihistamines, hormonal replacement therapy and androgen therapy—can worsen dry eye symptoms, too.10
According to DEWS II, 18 classes of drugs can negatively impact dry eye.12,15,22 Polypharmacy, where multiple medications are used concurrently, may also exacerbate dry eye symptoms. Researchers note that people older than 60 engage in polypharmacy at a rate of approximately 37%.15
The main strategy we try to employ with presbyopes that suffer contact lens discomfort related to dry eye disease (DED) or allergy is early detection and management. If we can detect clinical signs of ocular surface disease in the early stages, we can reduce patient symptoms, chair time and contact lens dropout.16,17 Prior to initiating lens wear for a new presbyope, look for clinical signs of meibomian gland dysfunction, lid wiper epitheliopathy, injection and any reduction in tear-film break-up time (TBUT).
Dry eye treatment plan discussions are often a fluid blend of clinical and therapeutic recommendations along with lifestyle modifications. Preservative-free artificial tears can reduce contact lens discomfort by reducing friction at the ocular surface that can lead to the initiation of the inflammatory cascade.18 Managing meibomian gland dysfunction with therapeutic warm compresses and lid hygiene effectively improves TBUT and lid health.18
Existing and new technologies like Lipiflow (Johnson & Johnson Vision), iLux (Alcon), TearCare (Sight Sciences) and intense-pulsed light (Lumenis and others) offer in-office opportunities for patients of all ages but especially those of mature age with conceivable ability or willingness to pay for these premium services. These hands-on options are also excellent considerations for those preferring a practitioner-involved process to service chronic lid margin disease.
Anti-allergy drops prior to or after lens removal, allergen avoidance and daily disposable lens wear are all modalities we employ to aid in the varying degrees of patients’ red, itchy eyes suffering from ocular allergies.
Objective improvement indicators cited in the DEWS reports that ODs should look for at the slit lamp include improved corneal and conjunctival staining, prolonged TBUT (improved over baseline) and improved quality of meibomian gland presentation with less capping and increase in lipid secretion quality.12,15,22 Clinical testing outside the slit lamp that indicates improvement would be a decrease in tear osmolarity detectable using a clinical osmometer (like that from TearLab).20 In 2014, researchers determined that osmolarity appears to be the best marker across all levels of disease severity as well as in different subtypes of dry eye disease.21
Restoring the function of the meibomian glands, improving the clinical corneal presentation and increasing tear film stability will allow for initial and long-term success.17 If we are able to identify the combination of therapy given each presbyopic patient’s clinical findings, ideally at an early phase, we can open their options up to different modalities of clear, stable vision at multiple ranges.
Contact Lens Options
Monovision contact lenses correct one eye for distance and the other for near ranges (or a modified version of this); patients who are able to tolerate the disparity do well without the need for additional near spectacle help. One disadvantage of monovision is the lack of depth perception and binocularity. We find monovision works preferably in patients who have notable one-eye dominance, amblyopia or other conditions that already limit binocularity.
Some patients are not good candidates for this option or are unable to adapt well; for them, consider multifocal contact lenses. The advantages of this modality are numerous, including the ability to provide simultaneous vision and binocular function at all ranges. Gas permeable, hybrid and scleral lenses with multifocal optics exist in several different designs, but can usually be incorporated into current user’s lenses. In the case of someone with keratoconus and prominent apical scarring, de-centering optics or varying zone sizes can be a triumph for these patients if we are able to adjust the optic zones accordingly where the impact of the scarring is minimized in a multifocal design.
Soft toric multifocals can be a solution for the unmet need of our presbyopic patients who have otherwise not had success secondary to their astigmatism. These manufacturers offer a broad range of parameters to correct or help significant toricity while performing well at near and intermediate ranges with stable vision. Beyond the standard available toric multifocals (Ultra Multifocal for Astigmatism, Bausch + Lomb), custom lens labs also offer a wide variety of soft lens designs and prescription parameters to tailor the optics for each patient.
Scleral lenses can also provide stable, clear vision while alleviating symptoms of dry eye disease.10 The vault of the lens over the cornea allows for a fluid reservoir (“moisture bath”) that acts to optically neutralize corneal irregularities and keep the ocular surface hydrated during wear.21
The DEWS report from 2013 recommended scleral lenses if other conservative treatment options such as artificial tears, lid therapy, topical pharmaceuticals or punctal plugs were inadequate in controlling ocular surface disease.22 This was upheld in DEWS II as a therapeutic consideration for patients with moderate to severe dry eye.12,15,22 Sclerals may help prevent or delay the patient from having procedures like amniotic membrane transplantation, tarsorrhaphy, mucous membrane or salivary gland transplant, or other lid surgeries.20-22 Their many advantages include protecting the ocular surface from further desiccation, providing continuous hydration to the cornea to repair underlying epithelial pathology (e.g., punctate corneal staining), allowing for best correctable vision and improving the patient’s quality of life.10,22,23
Scleral lenses also protect the ocular surface from irregular lid margins or, in cases of entropion that create exposure keratopathy and neurotrophic changes, lead to increased patient comfort.22,23 Research shows that scleral lens therapy promotes healing of surface epitheliopathy while reducing pain and photophobia.24,25 It is especially notable in cases refractory to standard treatments involving patients with ocular pain, burning, stinging, foreign body sensation, blurred vision and photophobia resulting from keratoconjunctivitis sicca secondary to chronic graft-vs.-host disease.23-25 No longer are reading glasses the only presbyopic option for contact lens wearers; scleral lenses present an opportunity to treat ocular surface disease while providing satisfying optical correction.9,25,26
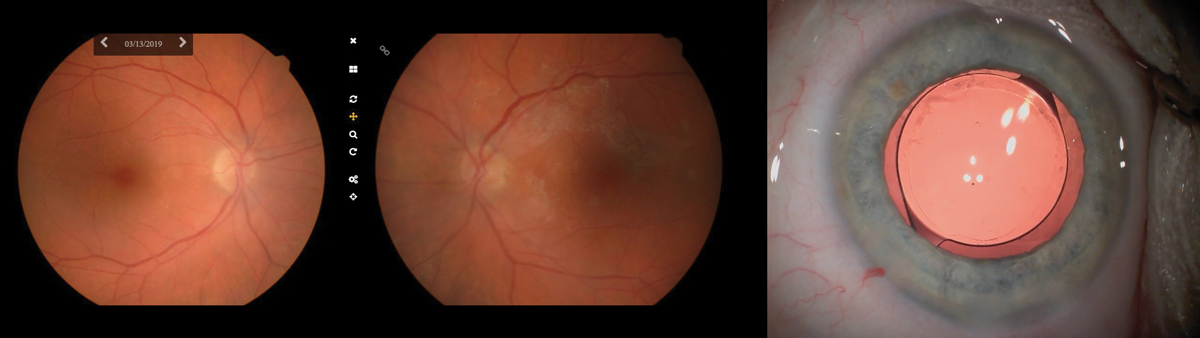 |
Left & middle: Fundus photos taken in the office of a 73-year-old woman sent for cataract surgery. These photos were sent along with the patient’s chart, helping the surgeon to visualize the underlying RPE mottling and surrounding atrophy most dense superior nasal to the fovea OD and a widespread epiretinal membrane OS. Right: We recommended a monofocal implant, seen here with retroillumination, as the retinal pathology would cause too much variability and thus undermine the success of a multifocal IOL. Click image to enlarge. |
Retinal Concerns
Preoperative management is a keystone of optometric care with regard to ocular and visual health, in addition to personality type and priorities. Notes to the surgeon that reflect patient habits, prior success with monovision or multifocal contacts or any clinical findings unique to the patient are helpful in the surgical process. Any pathology can result in decreased contrast sensitivity, decreased acuity and an unhappy patient experience.27-29 Document in detail epiretinal membranes, macular degeneration or any form of retinal pathology that may affect vision. As such, a proper dilated exam and well-written summary to the surgeon are imperative to success of any cataract surgery. A macular OCT before cataract surgery is very important and helps in the diagnosis of any suspecting and subtle pathology.27-29
Studies show no single type of IOL for these patients is completely without complications; therefore, we try to keep our explanations simple.27-29 Research also shows that it is not possible to infer a direct relationship between cataract surgery and age-related maculopathies; instead, we must use our clinical judgment and that of the surgeon to determine if cataract surgery will help the patients’ quality of life.27
Diffractive and refractive optics (or both) with multifocal IOLs cause light interference or light refraction through the implant, respectively.27-29 In patients with concurrent pathology, the way light is bent and strikes a diseased retina typically produces low rates of success with problems of dysphotopsia, worsening higher order aberrations and poor overall image resolution.27,28 Due to the fragile grasp we have on good vision with these patients, we typically recommend single vision (monofocal) lens implants to keep the visual system as balanced as possible.28,29
Any time we have any form of posterior segment pathology, visual potential will change as retinal disease progresses, which may be exacerbated by premium IOL options.27,29 We usually will discuss daily activities and what zone of clear vision each patient values the most. From there we can make sure to maximize their visual potential at their desired focal point in order to create a solid foundation for a positive visual outcome.
For those patients who have mild cataracts and mild retinal pathology, we may even steer them into a non-surgical option for the time being. This way we can still modify their prescription while letting mother nature run her course with possible progression of these concurrent etiologies.29
Prior Refractive Surgery
Here is another scenario where presbyopia gets the best of us, no matter what procedure we had to correct our distance vision in the past: “Doc, I had LASIK so I wouldn’t need glasses for distance; now you are telling me I need them again to read? You must be joking… right?”
According to the refractive surgery council, the number of refractive surgery cases has grown just over 6% since 2017 alone.30 This is a true test to the advances in technology and high success rates for the industry, though it does affect our patients’ response to presbyopia and tends to complicate matters when calculating IOL powers.30-32 Because of the improved technology and high success rates, these patients are so used to seeing well that any change in vision will be noticeable, putting more pressure on the optometrist and ophthalmologist for superior visual outcomes without spectacle correction.31,32
The best thing to do here is to keep our patient’s thinking as positive as possible and manage their expectation for all viewing distances. Highlight how their experience is a lot easier to manage post refractive surgery vs. prior. It has been a blessing that they have seen so well for so long, reiterating that they only need to wear correction for part of the day because their distance vision is still so clear.
The goal of any lenticular implant is to maximize clear vision at the patient’s specific desired ranges while reducing glare and minimizing post-surgical distortion.31,32 Patients with previous refractive surgery are already more likely to have a higher risk for postoperative dryness and higher-order aberrations.31,32 While there is no cure-all implant for our patients, historical refractive data is very important for the surgeon prior to IOL calculation.
Just as presbyopia motivates patients into the exam chair, it can also motivate them to put their trust in their eye care providers. The importance of dialogue cannot be stressed enough; simply knowing how to talk to the patient in your chair can be the difference between management success and failure. Knowing your audience is indispensable, and using this can help patients better absorb the science of presbyopia correction. The solutions are as ever changing as the problem itself and this realization is imperative to our patient’s progress.
There is not one right answer when satisfying near vision demands, but knowing the process and how to manage each type of presbyopic patient will spell continuous financial and practice growth for years to come.
Maj. Luft practices at Towne Lake Eye Associates in Woodstock, GA. He is a Fellow of the American Academy of Optometry.
Dr. Barbush practices at Levin Eye Care in Baltimore, MD. He is an Adjunct Assistant Clinical Professor and preceptor for SUNY and Salus Colleges of Optometry.
1. Fricke T, Tahhan N, Resnikoff S, et al. Global prevalence of presbyopia and vision impairment from uncorrected presbyopia. Ophthalmol. 2018;125(10):1492–9. 2. Mancil G, Bailey I, et al. Optometric Clinical Practice Guideline Care Of The Patient With Presbyopia. www.aoa.org/documents/optometrists/CPG-17.pdf. 2010. Accessed June 1, 2020. 3. Charman N. Developments in the correction of presbyopia: spectacle and contact lenses. Ophthalmic and Physiologic Optics. 2014;34(1):8-29. 4. Alvarez T, Kim E, Granger-Donetti B. Adaptation to progressive additive lenses: potential factors to consider. Sci Rep. 2017;7(1):2529. 5. Gray L. The prescribing of prisms in clinical practice. Graefes Arch Clin Exp Ophthalmol. 2008:246(5):627-9. 6. Meister, D. Understanding Prisms In Lenses. 18 February 2014. experiencevelocity.com/static_exentriqdotcom/documents/Zeiss_83466/825b9e6c-ca80-490f-9c2b-e87b3ecc2612.pdf. 7. Cook P. Prisms and progressives. 20/20. 2013;40(12):66-72. www.2020mag.com/article/prisms-and-progressives. 8. Antony J. Prisms in Clinical Practice. Kerala Journal of Ophthalmology. 2017; 29(2): 79-85. 9. Barnett M. Multifocal scleral lenses. Contact Lens Spectrum. www.clspectrum.com/issues/2015/december-2015/multifocal-scleral-lenses. December 1, 2015. Accessed April 9, 2020. 10. Barnett M. 10 tips to enhance scleral contact lens success. Optometry Times. www.optometrytimes.com/article/10-tips-enhance-scleral-contact-lens-success/page/0/4. June 20, 2017. Accessed April 9, 2020. 11. Employment Projections. Civilian labor force by age, sex, race, and ethnicity. U.S. Bureau of Labor Statistics. www.bls.gov/emp/tables/civilian-labor-force-summary.htm. September 4, 2019. Accessed April 25, 2020. 12. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334–65. 13. Coles‐BC, Sulley A, Young G. Management of digital eye strain. Clin Exp Optom. 2019;102(1):18-29. 14. Chang C. Presbyopia aggravates dry eye disease. J Clin Exp Ophthalmol. 2017;8:3(Suppl). 15. Craig J, Nichols K, Akpek E, et al. TFOS DEWS II Definition and classification report. The Ocular Surface. 2017;15(3):276-83. 16. Gu Q, Dillon CF, Burt VL NCHS Data Brief. Prescription drug use continues to increase: U.S. prescription drug data for 2007-2008. 2010;(42):1-8. 17. Markoulli M, Kolanu S. Contact lens wear and dry eyes: challenges and solutions. Clin Optom (Auckl). 2017;9(2):41–8. 18. Olson M, Korb D, Greiner J. Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact Lens. 2003;29(2):96–9. 19. Nichols K, Foulks G, Bron A, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis. Sci. 2011;52(4):1922-9. 20. Urgacz A, Mrukwa E, Gawlik R. Adverse events in allergy sufferers wearing contact lenses. Postepy Dermatol Alergol. 2015;32(3):204–9. 21. Haines L. Scleral lens use in dry eye syndrome. Contact Lens Update. www.contactlensupdate.com/2017/07/26/scleral-lens-use-in-dry-eye-syndrome. July 26, 2017. Accessed April 10, 2020. 22. Foulks GN, et al. 2007 Report of the International Dry Eye Workshop (DEWS). The Ocular Surface. 2007; 5(2):114. 23. Harthan JS, Shorter E. Therapeutic uses of scleral contact lenses for ocular surface disease: patient selection and special considerations. Clin Optom (Auckl). 2018;10:65–74. 24. Takahide K, Parker PM, Wu M, et al. Use of fluid-ventilated, gas-permeable scleral lens for management of severe keratoconjunctivitis sicca secondary to chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(9):1016-21. 25. Norman C. Prescribing for presbyopia. Contact Lens Spectrum. www.clspectrum.com/issues/2017/july-2017/prescribing-for-presbyopia. July 1, 2017. Accessed April 24, 2020. 26. Gall R, Wick B, Bedell H. Vergence facility: establishing clinical utility. Optom Vis Sci. 1998; 75(10): 731-742. 27. Casparis H, Lindsley K, Kuo I, et al. Surgery for cataracts in people with age-related macular degeneration. Cochrane Database Syst Rev. 2017;2(2):CD006757. 28. Grzybowski A, Wasinska-Borowiec W, Alio J, et al. Intraocular lenses in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2017;255(9):1687–96. 29. Lamoureux E, Hooper C, et al. Impact Of cataract surgery on quality of life in patients with early age-related macular degeneration. Optom Vis Sci. 2007;84(8)683-8. 30. Number of LASIK surgeries in the United States from 1996 to 2020 (in 1,000). Statista. www.statista.com/statistics/271478/number-of-lasik-surgeries-in-the-us. July 18, 2016. Accessed June 10, 2020. 31. Patel R, Karp C, Yoo S, et al. Cataract surgery after refractive surgery. Int Ophthalmol Clin. 2016;56(2):169–180. 32. Savini G, Hoffer K. Intraocular lens power calculation in eyes with previous corneal refractive surgery. Eye and Vis. 2018; (5):18. |

