 |
A 16-year-old male presented with blurry vision OU. His best-corrected visual acuity (VA) was 20/40 OD and 20/50 OS. He had an unremarkable birth history and no known medical conditions. IOP was 19mm Hg OD and 18mm Hg OS, extraocular motilities were full in all gazes, confrontation visual fields were full and pupils were equally round and reactive to light. Anterior segment exam revealed inferocentral corneal thinning, steepening and Vogt’s striae OS>OD with anterior stromal scarring OS.
Take the Retina Quiz
1. What is the most likely diagnosis for this patient’s posterior segment?
a. Central serous retinopathy.
b. Exudative peripapillary neovascular membrane.
c. X-linked juvenile retinoschisis.
d. Optic disc pit maculopathy (ODP-M).
2. Which modality is most useful for detecting this maculopathy?
a. B-scan ultrasound.
b. Fluorescein angiography.
c. Fundus autofluorescence.
d. OCT.
3. Which is not a proposed source for this patient’s maculopathy?
a. Vitreous humor.
b. Cerebrospinal fluid.
c. Vitreoretinal traction.
d. All of the above have been proposed.
4. Which is not considered to be a reasonable management option?
a. Observation.
b. Oral corticosteroids.
c. Macular buckle.
d. Pars plana vitrectomy (PPV).
5. What is the general prognosis for similarly presenting patients?
a. Excellent, 20/20 in nearly all cases.
b. Good, 20/70 or better in most cases.
c. Poor, 20/200 or worse in many cases.
d. Very poor in many cases.
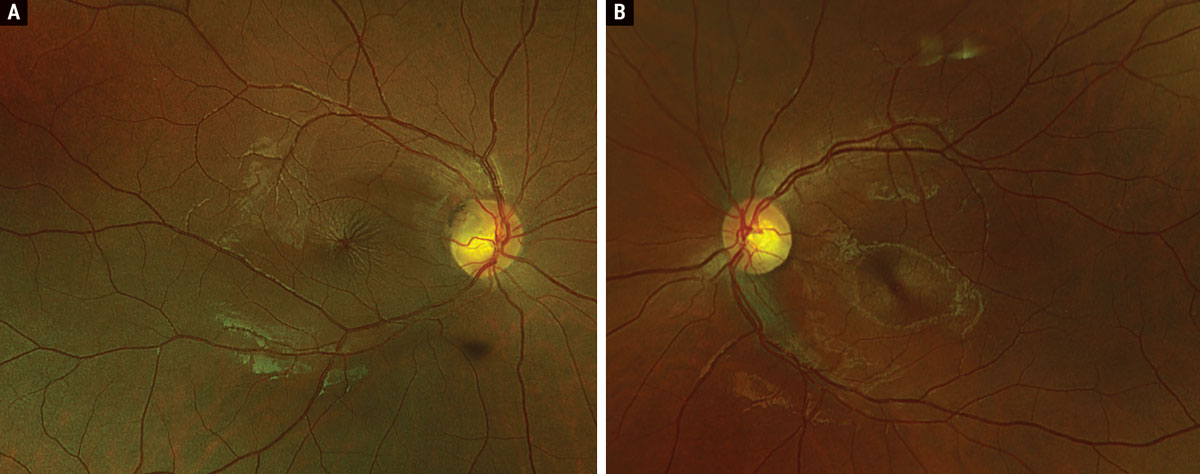 |
Fig. 1. Optos widefield fundus photography of the right (A) and left (B) eyes. Click image to enlarge. |
Diagnosis
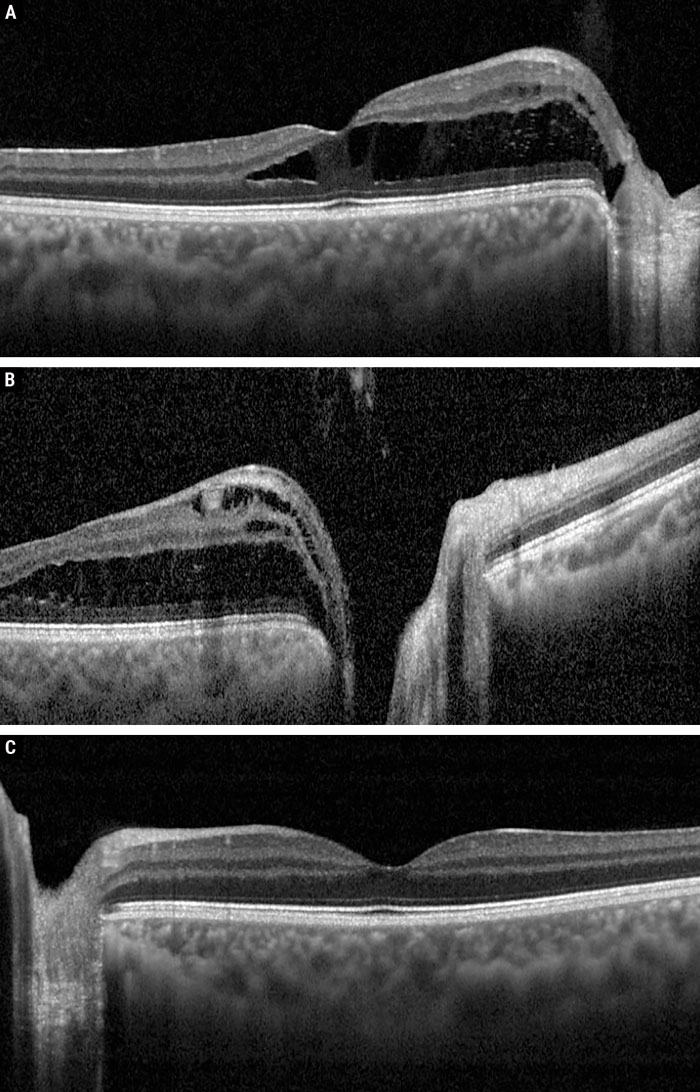
Regarding the anterior segment status, corneal topography confirmed a diagnosis of keratoconus. Careful examination of the fundus revealed a superotemporal optic pit OD with fluid extending into the superonasal macula and a central spoke-like appearance of the fovea; OS was normal with absence of an optic pit (Figure 1). OCT confirmed the presence of intraretinal (both inner and outer retinal) fluid extending from the pit into the fovea OD with no fluid present OS (Figure 2). A diagnosis of ODP-M was made.
ODPs are rare congenital optic nerve anomalies that were first described by Wiethe in 1882.1,2 The reported incidence is less than one in 10,000 and over 85% are noted to be unilateral in presentation with no racial or sex predilections.1,2 The presence of optic disc pits appears to be sporadic with no associated genetic variant.1,3 An acquired etiology has also been described in patients with (normal tension) glaucoma, producing a peripapillary retinoschisis appearance on OCT.4
Clinically, ODPs present as gray-brown, round-oval depressions found most frequently at the temporal or inferotemporal disc margin measuring approximately one-eighth to one-fourth disc diameter; central and nasal pits are less likely to produce maculopathy.1,3,5,6 Affected patients are generally asymptomatic in the absence of maculopathy, though reports suggest that ODP-M occurs in up to 75% of patients with ODP.1,3 Patients with ODP-M are most likely to present during the third to fourth decade of life (mean age of 31 years) with an average VA of 20/70 or worse.1,7 Serous retinal detachments are generally confined within the temporal arcades.2
While the exact pathophysiology of ODP-M is not well understood, the leading theories are a communication between the vitreous cavity and subretinal space, between the subretinal and subarachnoid spaces or a three-fold vitreous-subretinal-subaracahnoid fistula.4 Gass reviewed a histopathological specimen of an enucleated globe with ODP-M and found a somewhat fibrous capsule over the pit, theorizing this could present a barrier into the intra- and subretinal spaces.4 As he also was unable to identify vitreous glycosaminoglycans within the optic pit or the macular fluid, he postulated that cerebrospinal fluid would be most likely to enter the subretinal space through bypassing the pit’s fibrous capsule and approaching either between the capsule and scleral rim or by entering directly beneath invaginated neuroectodermal tissue.1,4
The hypothesis of vitreous subarachnoid fistulization was supported by documented migration of silicone oil from the vitreous cavity into both the subretinal space and intracranial cavity.1,4 There is also a consensus that vitreous traction likely plays a mechanistic role in the development of maculopathy, suggesting that treatments targeted at relieving traction may subsequently resolve the serous detachments.1,2
OCT is perhaps the most useful imaging modality for evaluating these patients, as fluid generally accumulates to produce a schisis-like cavity within the inner retina first, followed by the outer retina; this correlates with the clinical spoke-like appearance.2 However, there are reports of patients presenting with combined outer-retinal schisis and subretinal fluid, notably without inner-retinal schisis.2 It has been suggested that chronic outer-retinal schisis eventually results in lamellar hole formation, which provides an alternative pathway for the accumulation of subretinal fluid.1,2,8
 |
|
Fig. 2. OCT raster through the OD fovea (A), OD optic nerve (B) and OS fovea (C). Click image to enlarge. |
Management
Controlling ODP-M is challenging, and numerous therapeutic interventions have been attempted with variable success, indicating there is no standard of care. Oral corticosteroids have been trialed without success.1 Retinal photocoagulation has been applied in both an arcuate pattern temporal to the disc as well as circumferential to the disc with intent to create chorioretinal adhesions to barricade the pit from the macula with mixed results regarding efficacy and consistency.1,4,5,7
Intravitreal gas tamponade has also been tried in isolation and in combination with retinal photocoagulation or PPV, both serving to relieve vitreoretinal traction.1,2,7,8 Other surgical ideas that have been explored include macular buckling (technically difficult with steep learning curve) and inner-retinal fenestrations, which have shown moderate success rates.2,7 Gass proposed an algorithm for ODP-M of initial observation for at least one month, followed by one to two applications of retinal photocoagulation and ultimately intravitreal gas tamponade with or without PPV.5 Overall, PPV has a favorable safety profile, though no therapeutic approach has been shown to be uniformly successful.2,5 A prospective trial would be useful in comparing surgical options to determine an algorithmic approach for managing these patients.
Prognosis
Unfortunately, it is relatively poor, with many patients suffering irreversible VA loss of 20/200 or worse, especially in cases with chronic detachment.7 It has been argued that earlier intervention may presumably yield greater visual recovery.2,7 Our patient was recommended observation given the good presenting VA. Furthermore, his ODP-M is confounded by keratoconus, making it difficult to discern the visual significance of his maculopathy.
Retina Quiz Answers
1: d, 2: d, 3: d, 4: b, 5: c
Dr. Aboumourad currently practices at Bascom Palmer Eye Institute in Miami. He has no financial disclosures.
1. Kalogeropoulos D, Ch’ng SW, Lee R, et al. Optic disc pit maculopathy: a review. Asia Pac J Ophthalmol (Phila). 2019;8(3):247-55. 2. Shah SD, Yee KK, Fortun JA, Albini T. Optic disc pit maculopathy: a review and update on imaging and treatment. Int Ophthalmol Clin. 2014;54(2):61-78. 3. Brown GC, Shields JA, Goldberg RE. Congenital pits of the optic nerve head. II. Clinical studies in humans. Ophthalmology. 1980;87(1):51-65. 4. Gass JD. Serous detachment of the macula. Secondary to congenital pit of the optic nervehead. Am J Ophthalmol. 1969;67(6):821-41. 5. Agarwal A, Gass JDM. Gass’ Atlas of Macular Diseases. 5th ed. London: Elsevier; 2011. 6. Gordon R, Chatfield RK. Pits in the optic disc associated with macular degeneration. Br J Ophthalmol. 1969;53(7):481-9. 7. Chatziralli I, Theodossiadis P, Theodossiadis GP. Optic disc pit maculopathy: current management strategies. Clin Ophthalmol. 2018;12:1417-22. 8. Christoforidis JB, Terrell W, Davidorf FH. Histopathology of optic nerve pit-associated maculopathy. Clin Ophthalmol. 2012;6:1169-74. |

