 |
A 57-year-old African-American gentleman presented to the office for a routine diabetic eye exam. He had no complaints; his general practitioner asked him to obtain a funduscopic examination because he had systemic diabetes and hypertension. His systemic history was remarkable for well-controlled hypertension and type 2 diabetes. He denied allergies of any kind.
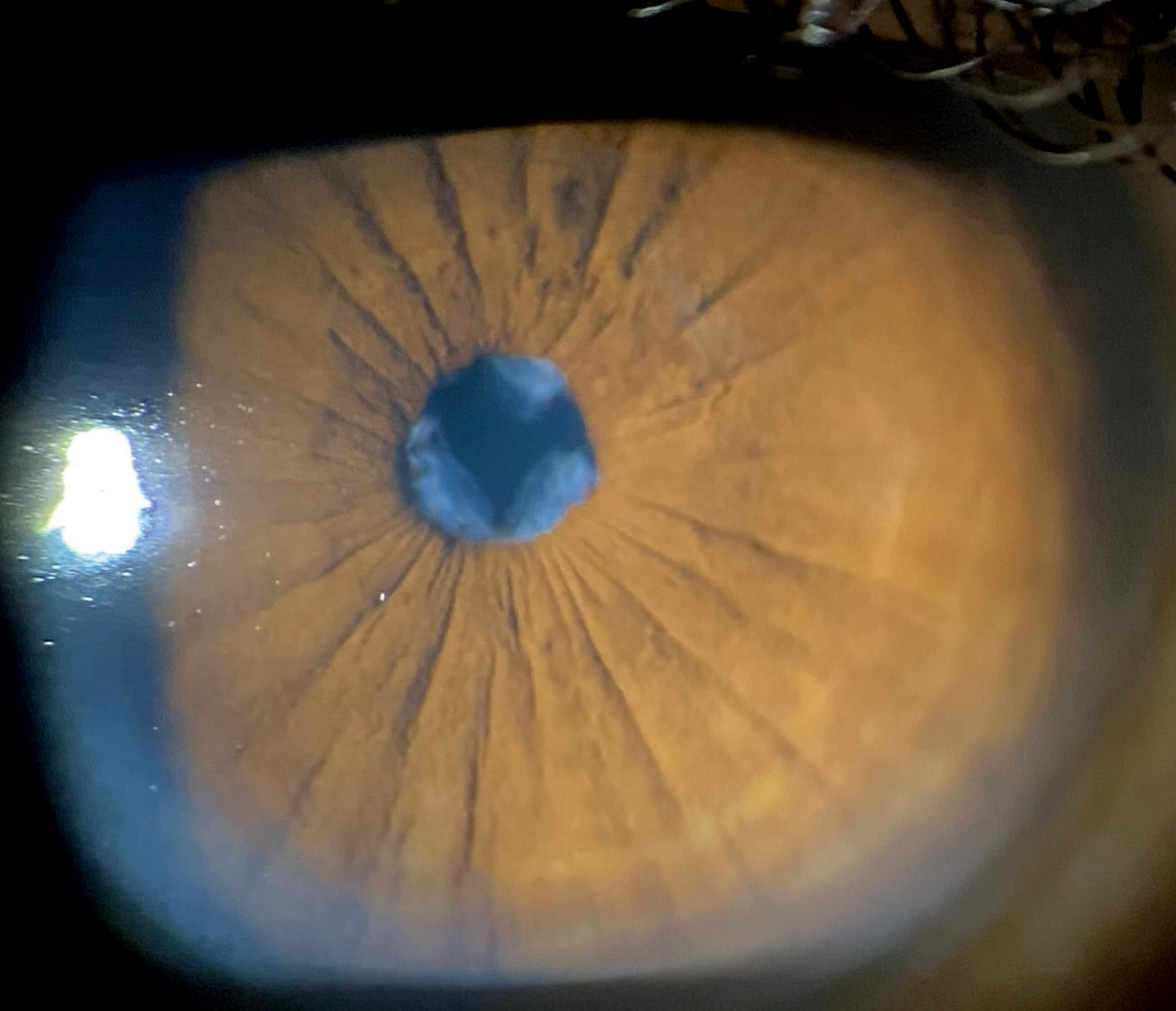
His best-corrected entering visual acuities were 20/30 OU at distance and near. His external exam was normal and there was no afferent pupil defect. The pertinent anterior segment finding discovered during the biomicroscopic exam is demonstrated in the photograph. Goldmann applanation tonometry measured 15mm Hg OU. The dilated fundus findings were normal peripherally and centrally with normal nerves and maculae.
Additional studies included inspection of the pupillary margin to ensure there was neither posterior synechiae (PS) nor iris neovascularization, detailed exam of the corneal endothelium for keratic precipitates or Krukenberg’s spindles, gonioscopy to look for peripheral anterior iris synechiae (PAS) or angle dysgenesis, inspection of the iris stroma to ensure there was no evidence of inflammatory cells (Busacca nodules, Koeppe nodules) and photodocumentation.
 |
|
A wispy membrane can be seen through the pupil on biomicroscopy. Click image to enlarge. |
Iris Issues
The diagnosis in this issue is iris persistent pupillary membrane (PPM). After birth, remnants of the iris pupillary membrane may persist.1-3 PPM are a common congenital anomaly seen in 95% of neonates.3,4
Embryologically, the iris forms from mesodermal tissue and in its infantile stages of development is known as the pupillary membrane.3 The primordial iris membrane is composed of vessels derived from anterior ciliary arteries and mesenchyme ventral to the crystalline lens.3 Behind the lens, the posterior hyaloid vessels (known as the tunic vasculosa lentis) form a network around the posterior lens capsule. These vessels extend anteriorly to anastomose with the network of vessels in the pupillary membrane.3 This structure is programmed to regress sometime around the sixth month of life and, under normal circumstances, disappears completely by the eighth month of gestation.3 It is a failure of cellular activities that result in the interruption of regression of the pupillary membrane that leads to PPM.3
This phenomenon may occur unilaterally or bilaterally.1-3 Failure of involution of the posterior hyaloid system leads to the development of a persistent hyperplasic primary vitreous.3,5
PPM tissue may be variably pigmented or unpigmented and is often contiguous to the iris collarette, but it doesn’t have to be.1-3 If significant in volume or central location, interrupting the visual axis, the formation of deprivation amblyopia is plausible.1 In rare instances, if located in or near the angle of the eye, egress of aqueous is slowed and secondary open-angle glaucoma may occur. Interestingly, if the affected individual has any significant refractive error, a PPM that interdigitates with the pupillary opening may provide a pinhole effect, which permits better uncorrected seeing.3
Accessory iris membrane (AIM) is a rare congenital ocular anomaly that presents as iris tissue that often completely covers the pupillary area.3,4 AIM resembles normal iris tissue in color, pattern and thickness but has no muscular activity.3 In most instances AIM are bilateral, associated with deprivation amblyopia, anterior polar cataract and varying forms of strabismus.3,4
Other differential diagnoses may include posterior synechiae from chronic ocular inflammation (random attachment, evidence of keratic precipitate), Axenfeld-Rieger syndrome (ectropion uvea, displaced Schwalbe’s line), Peter’s anomaly (corneal lenticular connections) and iridocorneal endothelial syndrome manifestations like Chandler syndrome (persistent corneal edema]), essential iris atrophy (polycoria, ectropion uvea), Cogan-Reese or iris nevus syndrome pedunculated iris nodules).4,6
In most instances, no intervention is required. If there are visual axis concerns, chronic mydriatic therapy with cylopentolate 1% or 2% can be attempted to reorient the intruding tissue or disrupt it.2 YAG laser membranectomy has been used to effectively disrupt and remove the membrane.3 The principal complications of this modality include blood vessel disruption—which can lead to hyphema and all of its complications (iritis, raised intraocular pressure, corneal blood staining—cataract formation, chronic iritis and pigment dispersion with or without secondary open-angle glaucoma.3
In the event the aforementioned interventions fail and there are ongoing or worsening issues with aqueous egress or deprivation amblyopia, surgical removal can be accomplished via pupillary meiosis and vitreous scissors.2 Surgical removal is not free of risks, which include: need for general anesthesia, the possibility of provoking intraoperative bleeding, the possibility of provoking intraocular infection and iatrogenic lens damage with stimulus to early cataract formation.2
If uncorrected refractive error is exposed following PPM removal (removal of the pinhole effect), spectacle correction may be required. If amblyopia is possible because of the location, density and chronicity of the PPM, it must be identified and then rehabilitated in the normal fashion.
This patient was photodocumented, educated and will be monitored biannually.
Dr. Gurwood is a professor of clinical sciences at The Eye Institute of the Pennsylvania College of Optometry at Salus University. He is a co-chief of Primary Care Suite 3. He is attending medical staff in the department of ophthalmology at Albert Einstein Medical Center, Philadelphia. He has no financial interests to disclose.
1. van Poppelen NM, Vaarwater J, Mudhar HS, et al. Genetic background of iris melanomas and iris melanocytic tumors of uncertain malignant potential. Ophthalmology. 2018;125(6):904-12. 2. Allegrini D, Montesano G, Pece A. Optical coherence tomography angiography of iris nevus: a case report. Case Rep Ophthalmol. 2016;7(3):172-8. 3. Shields JA, Sanborn GE, Augsburger JJ. The differential diagnosis of malignant melanoma of the iris. A clinical study of 200 patients. Ophthalmology. 1983;90(6):716-20. 4. Shields CL, Kaliki S, Hutchinson A, et al. Iris nevus growth into melanoma: analysis of 1611 consecutive eyes: the ABCDEF guide. Ophthalmology. 2013;120(4):766-72. 5. Shields CL, Shields PW, Manalac J, Jumroendararasame C, Shields JA. Review of cystic and solid tumors of the iris. Oman J Ophthalmol. 2013;6(3):159-64. 6. Nichols JC, Amato JE, Chung SM. Characteristics of Lisch nodules in patients with neurofibromatosis type 1. J Pediatr Ophthalmol Strabismus. 2003;40(5):293-6. 7. Shaikh N, Kumar V, Venkatesh P. Iris nodules in Fuchs heterochromic iridocyclitis. Indian J Ophthalmol. 2019;67(8):1339. 8. Postolache L, Parsa CF. Brushfield spots and Wölfflin nodules unveiled in dark irides using near-infrared light. Sci Rep. 2018;8(12):18040. 9. Mensink HW, Vaarwater J, de Keizer RJW, et al. Chromosomal aberrations in iris melanomas. Br J Ophthalmol. 2011;95(3):424-8. 10. Sandinha MT, Kacperek A, Errington RD, Coupland SE, Damato B. Recurrence of iris melanoma after proton beam therapy. Br J Ophthalmol. 2014;98(4):484-7. |

