Practical Matters in Myopia Management - 3rd EditionWith an expanding arsenal of information, myopia can be a puzzling area of care. In this third annual Review of Optometry supplement, experts offer solutions backed by science to help you manage this growing patient population. Click here to download a PDF or here to read the digital edition. Check out the other articles featured in this supplement: |
Change can take time. Bloodletting, or the practice of withdrawing blood from an ill person thought to be suffering from an imbalance of “humors,” lasted from 1000 BCE to the 19th century. In eye care, the first eyeglass corrections for myopia date back to the 15th century and, even today, single-vision eyeglass corrections are still undoubtedly the most common correction for myopic refractive error.
There is a sense that medical science is evolving more rapidly now that we are in the “information age.” But does this apply to myopia, which has classically been characterized as a “simple” refractive error with treatment merely requiring a compensator? There are now clear reasons to consider myopia a disease, and prevention and control in children an urgent need. If things continue in the direction they are going, myopia is estimated to affect five billion people by the year 2050, with one billion of those being high myopes.1 There is a correlation between each diopter of myopia or, more precisely, each millimeter of axial elongation, with vision impairment.2,3 The correlation is driven by the relationship of myopic eye growth with the risk of glaucoma, cataracts, retinal tears/detachments and myopic macular degeneration. The upshot is that poorly controlled myopia progression is effectively the most significant factor in preventable visual impairment and blindness globally.4
There is broad agreement by various professional bodies that children with myopia and those identified to be at risk for myopia should be identified, and their parents should be advised of the risks of myopia progression and options to minimize it.5,6 There are several quite effective treatments to delay the onset and slow progression of myopia.7 However, arguments remain regarding who to treat, when to treat them, how to evaluate the effectiveness of the chosen treatment, when to swap one treatment for another or when to select additive therapy.
Below, we’ll offer optometrists guidance on navigating these treatment decisions with an emphasis on options currently available in the US. Additionally, we’ll provide insights on myopia control methods approved overseas to give ODs in the States an idea of where and how these interventions will eventually fit into their practices.
Lifestyle Recommendations
Before we explore considerations for approved therapeutics for myopia control, it’s important to highlight that regardless of where you practice in the world, every eyecare clinician can recommend lifestyle modifications and health-promoting habits to patients with myopia. There are no product approvals for regulators to vet, no equipment for clinicians to purchase and no costs for families to bear—financially, at least. Kids may dissent from that view when asked to put down their phones and go outside.
Here’s what the current research tells us about certain daily habits that relate to the prevention or progression of myopia:
Outdoor time. There is clear evidence that increased outdoor time delays the onset of myopia and that the later the onset, the less likely high myopia will occur. Whether the outdoor time is effective in itself because of exposure to sunlight’s different wavelengths and luminance levels vs. indoor light or simply by reducing near work through changing habits, the results are the same. This is the most straightforward intervention that can be deployed at the youngest age, and it is simple, free, fun and has broader health benefits.
Near work/device use. It feels intuitively likely that near work causes myopia, though many studies have failed to prove it. This is perhaps due to difficulties characterizing near work behaviors via questionnaires—were the tools able to accurately quantify time spent, viewing distance(s), paper vs. device vs. type of device vs. screen size? Additionally, many studies fail to properly classify different types of young myopes in terms of their binocular vision. Even with inadequate and contradictory evidence, there is a building consensus that proper visual hygiene may be helpful.
• The World Health Organization suggests avoiding any screen time below the age of two years, with a limit of one hour between ages two and five.8
• The 20-20-20 rule (take a 20 second break every 20 minutes of close work to gaze at something at least 20 feet away) was suggested in the 1990s to alleviate digital eye strain.9 While it is easy to remember, it is not an anti-myopia recipe and has come under criticism in recent years.10
• A correlation has been found between close viewing distances for longer durations and myopia, supporting suggestions that children should increase their viewing distance, decrease their duration of near work and to take frequent breaks.11
Other lifestyle factors. Aside from time spent on near work, other health-related factors that may eventually be better proven to correlate to myopia development or progression include diets that lead to obesity and the effects of vitamin D and melatonin, each of which relate to outdoor time and the impact of good sleep habits.12 It’s safe to say, then, that counseling your patients with myopia to adopt healthier habits—such as spending more time outdoors, decreasing electronic device use and getting sufficient shut-eye each night—at the very least won’t do them any harm.
 |
|
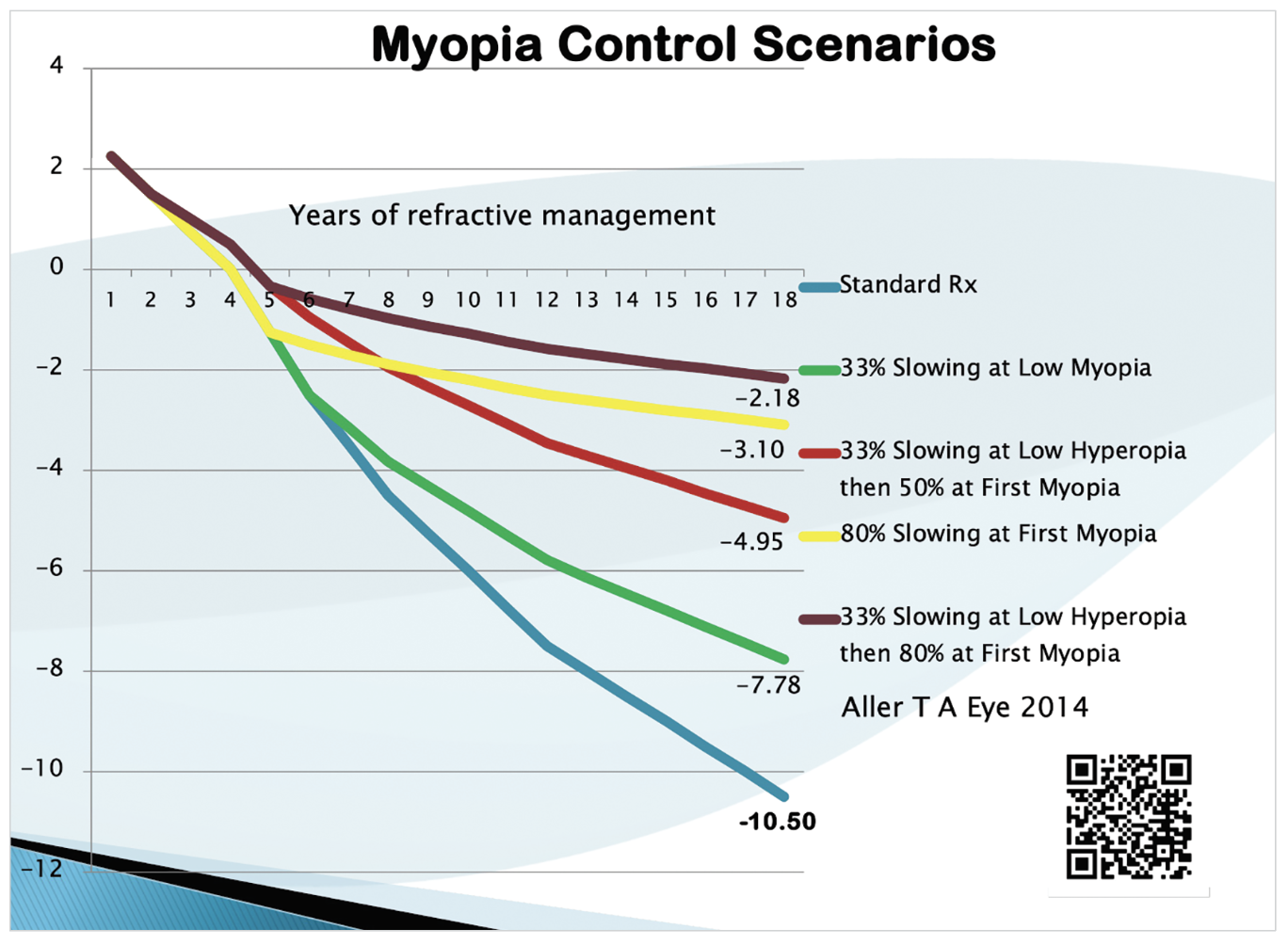
Fig. 1. How early in the myopia progression process an intervention is implemented, as well as the treatment type, can have a direct effect on refractive outcomes, as illustrated here.7 Click image to enlarge. |
Myopia Control Treatments in the US
Clinical interventions currently available for patients with myopia in the United States include low-dose atropine eye drops obtained through a compounding pharmacy, MiSight contact lenses (CooperVision), other EDOF multifocal soft contact lenses and orthokeratology.
When deciding how early to recommend one or more of these treatments, consider that axial elongation often increases most rapidly in the year before the official onset of myopia and that the rate of increase slows with age.13 Therefore, the most significant impact of treatment may result from the earliest implementation with the highest efficacy. Figure 1 illustrates this point by showing various refractive outcomes based on choices made as to how early in the myopia progression process an intervention is implemented, as well as by how different treatment choices with varied efficacies can also alter the final level of myopia.7
Below, we review the myopia management options approved or available off-label in the US and offer guidance on which to prescribe and when:
Atropine. These drops appear to reduce myopia progression via a scleral or retinal mechanism not related to their mydriatic or cycloplegic effects; however, the exact mechanism remains unknown. Below are some parameters and suggestions for atropine use in myopes based on current evidence and personal experience:
• Atropine effects are dose-dependent, including the intended result (higher doses result in slower progression), side effects (higher doses result in increasing glare and near blur) and rebound (higher doses result in greater increases in myopia progression when ceased abruptly).
• Balancing these factors, the most common current trend is to start treatment with 0.05% atropine once daily before bed. This dose may then be adjusted higher or lower depending on whether more efficacy is desired or to reduce side effects. A personal adjustment is to instill 0.05% drops Sunday through Thursday, leaving the weekends more comfortable for outdoor activities and promoting compliance, comfort and efficacy. With studies showing the effectiveness of once-weekly 1.0% atropine, as well as equal efficacy with every night vs. every other night dosing strategies, “weekend drug holidays” may also be effective.8,9
• An atropine study would be welcome with a strategic tapering strategy upon discontinuation of treatment intended to avoid an unnecessary rebound. However, there is one study that analyzed dose reductions within the study to look for rebound progression and reported none.14 A recent meta-analysis reviewed many atropine studies to try to better understand rebound myopia progression and ways to avoid it.15 While purposeful studies intended to avoid rebound myopia are still necessary, their conclusions include that rebound was strongest the younger the patient, the higher the myopia, the higher the dose and the shorter the treatment duration. Helpfully, they also discovered that when atropine was discontinued while other myopia control treatments were being used, no significant rebound was observed.
• There are several atropine formulations from different companies at various stages of development, including Vyluma (NVK002 0.01% atropine) whose new drug application was recently accepted by the FDA, Sydnexis (SYD-101 0.01% and 0.03% atropine) and Eyenovia, which has developed a microdosing spray for atropine (MicroPine) that can be dispensed at the push of a button.
These approvals, should they occur, and once their clinical findings are publicized, could change our current approach to atropine therapy for myopia.
Contact lenses. For children mature enough to handle the responsibilities of this treatment modality, MiSight myopic defocus daily disposable contact lenses (CooperVision) are FDA-approved in the US for the control of myopia progression in children. Other lens designs have approvals outside the country, such as the NaturalVue 1 Day multifocal (Visioneering Technologies), Mylo monthly replacement extended depth-of-focus lenses (Mark’ennovy) and Abiliti ortho-K lenses as well as Acuvue 1-Day Abiliti soft lenses (Johnson & Johnson). Biofinity D multifocals (CooperVision) with +2.50D add power were shown to effectively control myopia in the BLINK study, though without a CE mark at this time.16
US practitioners can, of course, use any available multifocal lens for myopia control on an off-label basis, though it’s always best to have good support in the literature for any off-label treatments.
Ortho-K lenses. The only orthokeratology lens with a CE mark for the control of myopia progression is the new Abiliti Overnight (Johnson & Johnson). However, efficacies in most ortho-K studies are in the 50% average range. Ortho-K is different from all other treatments in that standard designs not optimized for myopia control will create optical profiles that are correlated with the initial level of myopia. Thus, it has been shown that older designs or standard ortho-K designs will offer greater control of myopia, the higher the initial level of myopia.
Recent studies have shown that smaller treatment zones and even decentered treatment zones can control myopia better and in lower myopes as well. Studies have also shown that children with larger pupils have greater myopia control with ortho-K, which may explain the additive effects of 0.01% atropine with ortho-K.
There is a decent argument that since ortho-K and multi- or dual-focus soft lenses can be expected to control myopia with roughly equal efficacy, practitioners should select the treatment modality that best suits the patient’s lifestyle, as this will help maximize treatment compliance.
• High astigmatism, oblique astigmatism or myopia levels too high either for good vision or beyond the FDA-indicated levels, are likely to be poor choices for ortho-K based on expected visual performance. In the case of low to very low myopia, ortho-K can result in unwanted residual hyperopia or lesser control of progression.
• Spherical soft lenses and lenses with FDA-imposed prescription limits could also result in poor visual performance. However, the availability of custom multifocal toric lenses, as well as distance-centered multifocal hybrids with add powers up to +5.00D, means that a lens is available for any level of myopia or pre-myopia.
• Ortho-K may also be employed to partially correct higher levels of myopia; this has been shown to be quite effective, though daytime correction with spectacles would likely be necessary.17
Treatment Options Outside the US
The use of myopia control spectacles and light-exposure treatments to control the progression of myopia is commonplace in many regions outside of the US, including in Canada, Europe, Asia and Australia. Since it’s only a matter of time before these interventions become approved in the States, clinicians who treat this patient population must understand how they work, which patients will benefit and where they fit into myopia management protocols.
Here are some considerations for myopia control treatments not available in the US (yet):
Myopia control spectacles. Several of these are in use around the world today with proven efficacies in terms described by the mnemonic CARE (Cumulative Absolute Reduction in axial Elongation). Examples include SightGlass DOT (SightGlass Vision), Stellest (Essilor) and MyoSmart (Hoya). They all offer safe and useful myopia control in a range of situations:
• The inherent low risk of these lenses vs. contact lens or pharmaceutical interventions makes them a helpful option even before myopia becomes symptomatic—that is, in the plano to -0.75D range, where most families are hesitant about contact lens or pharmaceutical options.
• They can also be used in children too young for contact lenses, when parent concerns or child aversion precludes pharmaceuticals or if unacceptable side effects result from atropine dosages adjusted for efficacy.
• For maximum treatment efficacy, these lenses may need to be worn on a full-time basis. This may be less likely in the case of a low myope who may not perceive much visual benefit. Frame selection and proper centration of the lenses may also be critically important.
• Even though lens manufacturers charge a premium for the technology in the lens designs, they can still work out as the least expensive long-term myopia management option.18
Light exposure therapy. There are several emerging light-based treatments purported to slow myopia progression, including red-light (Eyerising International) and blue-light (MyopiaX, Dopavision) units. While red light should be inherently safer for eyes than blue light, the laser light levels produced by red-light units are many times greater. The myopia control effect of red light is thought to occur via a choroidal blood flow mechanism, whereas the blue-light effect is believed to occur via a dopamine pathway. Short-term efficacy results are promising for both, though one prominent concern is that long-term safety and efficacy remain uncertain.
To our knowledge, no FDA trials of light therapy devices are underway, so such interventions are not a realistic short-term prospect in the US.
Monitoring Progression
Every optometrist can do something to help control myopia progression in their patients. This may involve prescribing some options while referring to colleagues with specialty interests for others. However, practitioners specializing in myopia and committed to delivering the highest efficacy must have a way to measure axial length accurately.
Being able to measure changes in axial lengths to micron-level accuracy allows evaluation of treatment choices over short periods (by three months in most cases). Axial length measurement also facilitates the comparison of various scenarios in monocular trials. For example, which atropine concentration is optimal for this patient? Should they instill one drop or two, and for how many days per week? Additionally, observing axial length measurements across visits allows you to objectively assess the effect of changing from one soft lens design to another, one add power to another or one treatment zone diameter in ortho-K or a multifocal contact lens. Optical biometry makes these kinds of evaluations possible.
One hazard of frequent axial length measurements is that you may be detecting seasonal differences rather than treatment efficacy changes. If you want to think your treatment choice is brilliant, you would prescribe in May and re-evaluate in September, as that aligns with an expected seasonal slowing of myopia. The converse is also true with a treatment initiated in August and re-evaluated in January as myopia progresses more rapidly in the winter and/or the academic year.
Many optical biometers now include specific features important in myopia management. Being able to track changes over time, as well as being able to compare the observed changes to expected progression of myopia or axial length in both myopic populations and emmetropic populations, is incredibly effective for the practitioner in evaluating treatment effectiveness and communicating with patients and parents. While it is reasonable to assert that a treatment limiting axial elongation to expected emmetropic levels is effective, it’s also important to consider that many myopic children have already experienced more axial elongation than their eyes would have had should they never had developed myopia; therefore, allowing “normal, expected emmetropic” growth may still be excessive for an already elongated eye.
 |
|
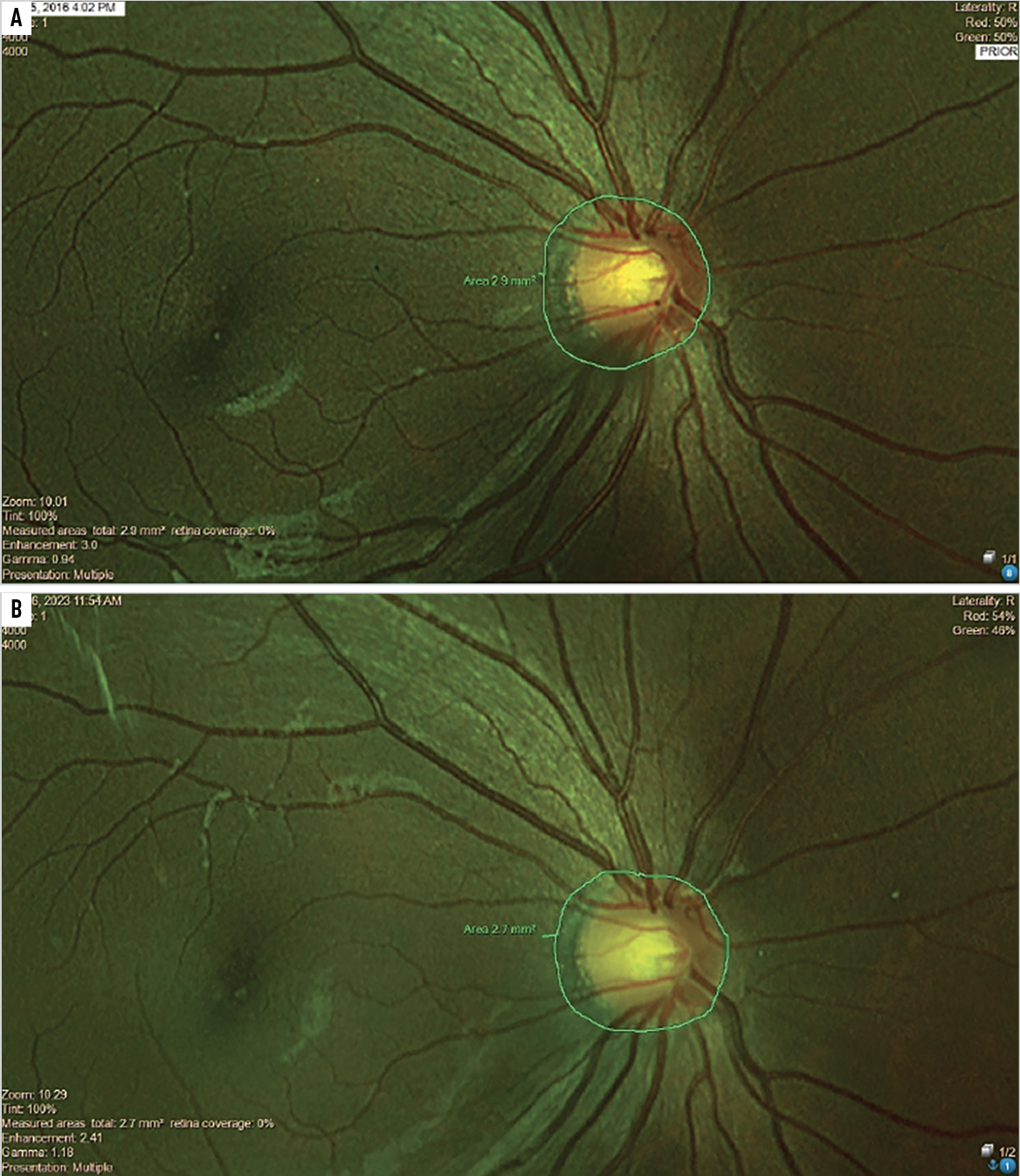
Fig. 2. This nine-year-old child with myopia was effectively treated with multifocal contact lenses for seven years, during which she had about -0.25D total myopia progression and stable axial lengths. The serial analysis of the area of peripapillary choroidal atrophy shows virtually no change from before treatment (A) to seven years later (B). Click image to enlarge. |
Takeaways
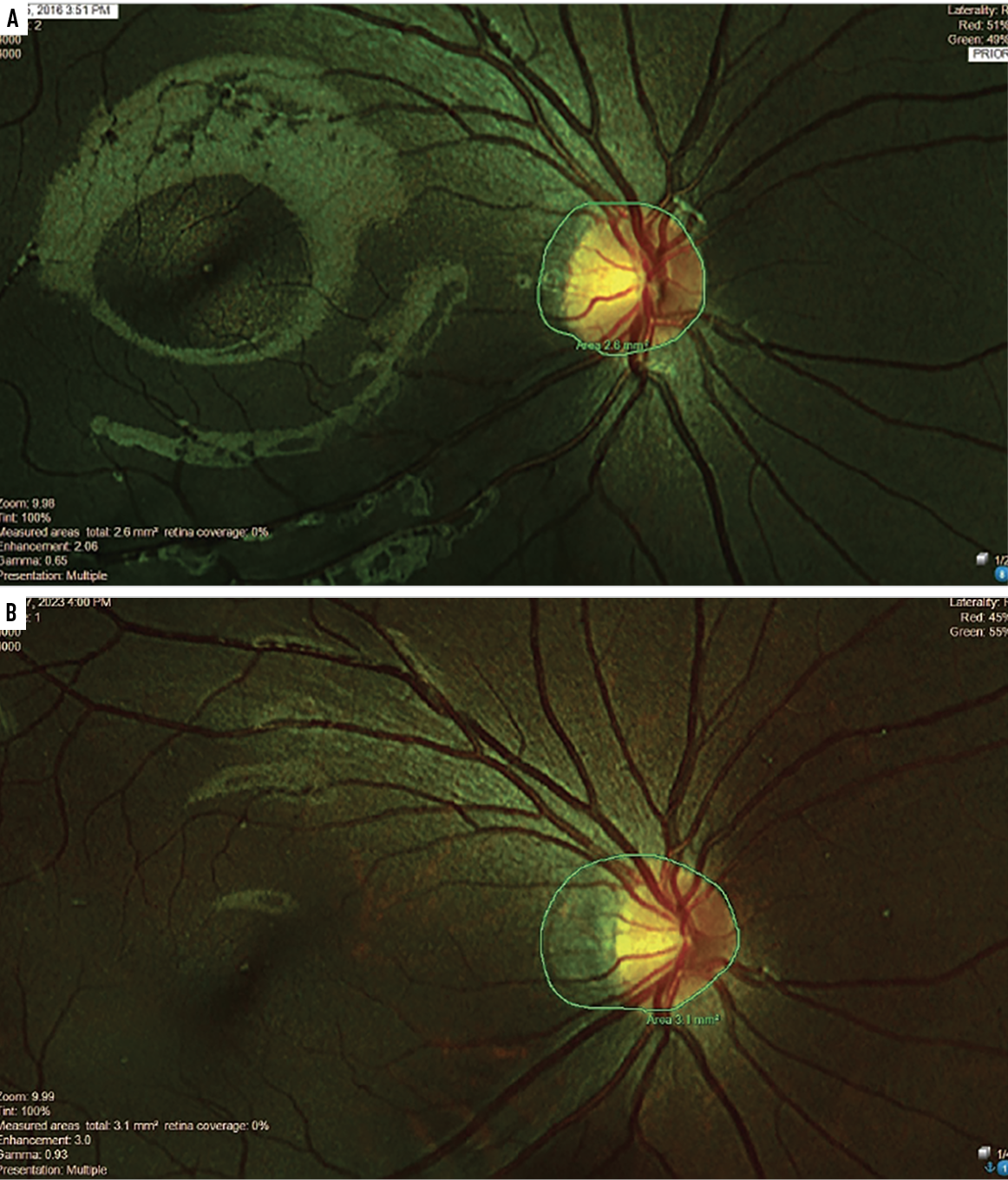
We’ll close with a tale of two nine-year-old Chinese-American female myopes, one of whom opted to manage her myopia with multifocal soft contact lenses and the other who refused effective treatments for seven years. The effectively treated child had about -0.25D total myopia progression and stable axial lengths while the child opting for a mix of standard single-vision spectacles and occasional progressive addition lenses increased in myopia by over -4.00D (axial lengths were not measured in this untreated child). What is striking and perhaps alarming in the fundus photos of each child is that serial analysis of the area of peripapillary choroidal atrophy shows virtually no change in the treated child (Figure 2). Meanwhile, there are marked increases in the area of atrophy in the untreated child (Figure 3). Not shown in these central photos is the development of extensive areas of white without pressure in the peripheral retina of this ineffectively treated child.
 |
|
Fig. 3. Contrary to the treated patient shown on page 12, this nine-year-old patient refused treatment for seven years and instead opted for standard single-vision spectacles and occasional progressive addition lenses. The area of atrophy appreciably increased from year two (A) to year nine (B). Click image to enlarge. |
Significant myopia progression should be considered a lifelong disease, with prevention, mitigation and treatment phases. The weight of expert opinion favors myopia control with considered choice(s) from the multiple, proven treatment options. There remains the need to craft and refine one’s professional protocols for managing myopia effectively.
Dr. Aller has been involved in myopia control research and development for over 30 years. He is a visiting scholar at UC Berkeley and a clinical advisor to many companies involved in myopia research and innovation. Dr. Aller is also a fellow of the BCLA and an associate member of the International Society of Contact Lens Specialists.
Dr. Fricke is an optometrist, researcher and international development practitioner and is currently the director of Research and Education at the Australian College of Optometry and National Vision Research Institute. He has held clinical, teaching, research, management and leadership roles in private, public, community health, hospital and refugee camp settings, including as director of the Brien Holden Foundation. His research has covered pediatric eye care, epidemiology, quality of life, access to eye care and health economics. He is an honorary senior fellow at the University of Melbourne and is completing a PhD in ophthalmic epidemiology at UNSW Sydney.
1. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-42. 2. Tideman JWL, Tedja MS, Wong KT, et al. Association of axial length with risk of uncorrectable visual impairment for europeans with myopia. JAMA Ophthalmol. 2016;134(12):1355-63. 3. Bullimore MA, Brennan NA. Myopia control: why each diopter matters. Optom Vis Sci. 2019;96(6):463-5. 4. Naidoo KS, Fricke TR, Frick KD, et al. Potential lost productivity resulting from the global burden of myopia: systematic review, meta-analysis and modelling. Ophthalmology. 2019;126:338-46. 5. Modjtahedi BS, Abbott RL, Fong DS, Lum F, Tan D; Task Force on Myopia. Reducing the global burden of myopia by delaying the onset of myopia and reducing myopic progression in children. Ophthalmology. 2021;128(6):816-26. 6. Modjtahedi BS, Ferris FL 3rd, Hunter DG, Fong DS. Public health burden and potential interventions for myopia. Ophthalmology. 2018;125(5):628-30. 7. Gifford KL, Richdale K, Kang P, et al. IMI - clinical management guidelines report. Invest Ophth Vis Sci. 2019;60(3):M184-203. 8. World Health Organization. WHO guidelines on physical activity, sedentary behaviour and sleep for children under 5 years of age. Geneva. 2019. 9. Akerman D. The 20-20-2 rule. Review of Myopia Management. Published September 30, 2020. reviewofmm.com/the-20-20-2-rule. Accessed July 1, 2024. 10. Klaver C, Polling JR, Erasmus Myopia Research Group. Myopia management in the Netherlands. Ophthalmic Physiol Optics. 2020;40(2):230-40. 11. Gajjar S, Ostrin LA. A systematic review of near work and myopia: measurement, relationships, mechanisms and clinical corollaries. Acta Ophthalmol. 2022;100(4):376-87. 12. Liu XN, Naduvilath TJ, Sankaridurg PR. Myopia and sleep in children-a systematic review. Sleep. 2023;46(11). 13. Mutti DO, Hayes JR, Mitchell GL, et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophth Vis Sci. 2007;48(6):2510-9. 14. Polling JR, Tan E, Driessen S, et al. A 3-year follow-up study of atropine treatment for progressive myopia in Europeans. Eye (Lond). 2020;34(11):2020-8. 15. Lee SH, Tsai PC, Chiu YC, Wang JH, Chiu CJ. Myopia progression after cessation of atropine in children: a systematic review and meta-analysis. Front Pharmacol. 2024;15:1343698. 16. Walline JJ, Walker MK, Mutti DO, et al. Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: the BLINK randomized clinical trial. JAMA. 2020;324(6):571-80. 17. Charm J, Cho P. High myopia-partial reduction ortho-k: a 2-year randomized study. Optom Vis Sci. 2013;90(6):530-9. 18. Fricke TR, Sankaridurg P, Naduvilath T, et al. Establishing a method to estimate the effect of antimyopia management options on lifetime cost of myopia. Br J Ophthalmol. 2023;107(8):1043-50. |

