 |
A 35-year-old Hispanic male presented with acute onset painless “cloudy vision” OD for four days. The patient recalled a similar event of transient monocular vision loss OD four months prior that was self-limiting within three hours that he never sought medical care for. Medical history was unremarkable. Entrance VA was 20/20 OD and OS, pupils were equally round and reactive without RAPD, and confrontation visual fields and extraocular motilities were full OU. IOP was 15mm Hg OD and 14mm Hg OS. Slit lamp examination was unremarkable.
Take the Retina Quiz
1. Which of the following is NOT a clinical feature present on exam?
a. Central retinal vein occlusion (CRVO).
b. Paracentral acute middle maculopathy.
c. Cilioretinal artery occlusion.
d. All of the above.
2. Regarding the imaging, which of the following is false?
a. There are multifocal paracentral hyperreflective bands in the inner nuclear layer (INL) and outer plexiform layer (OPL).
b. There are multifocal paracentral hyperreflective bands in the outer nuclear layer (ONL) and OPL.
c. There is peripapillary inner-retinal hyperreflectivity.
d. There is peripapillary retinal thickening present.
3. What is the most appropriate next step?
a. Emergent stroke work-up.
b. Intravitreal anti-VEGF agent.
c. Observation.
d. Outpatient hypercoagulable work-up.
4. What test should be performed at follow-ups for patients with recent ischemic CRVO?
a. Gonioscopy.
b. Fundus photos.
c. OCT retinal nerve fiber layer.
d. Electroretinogram.
5. Which of the following is NOT a risk factor for the patient’s presentation?
a. Cigarette smoking.
b. Lipoprotein A.
c. Oral contraception.
d. All of the above are risk factors.
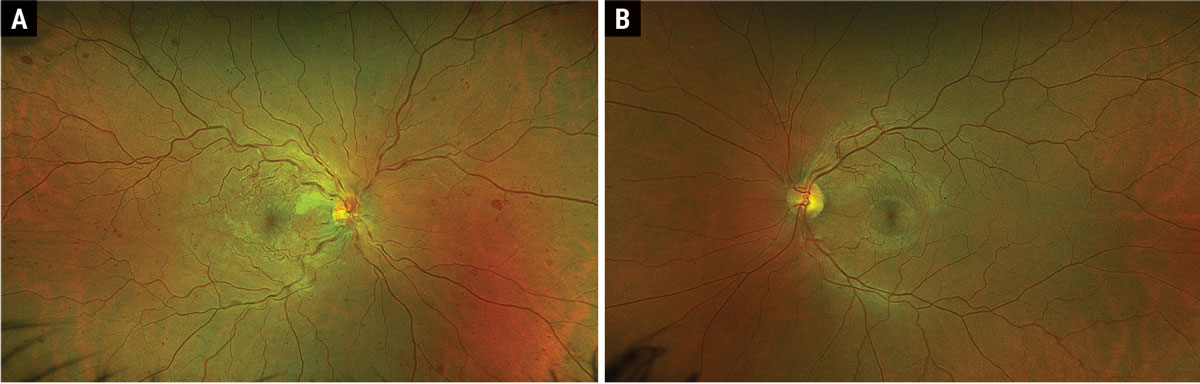 |
Fig. 1. Optos ultrawidefield fundus photograph of OD (A) and OS (B). Click image to enlarge. |
Diagnosis
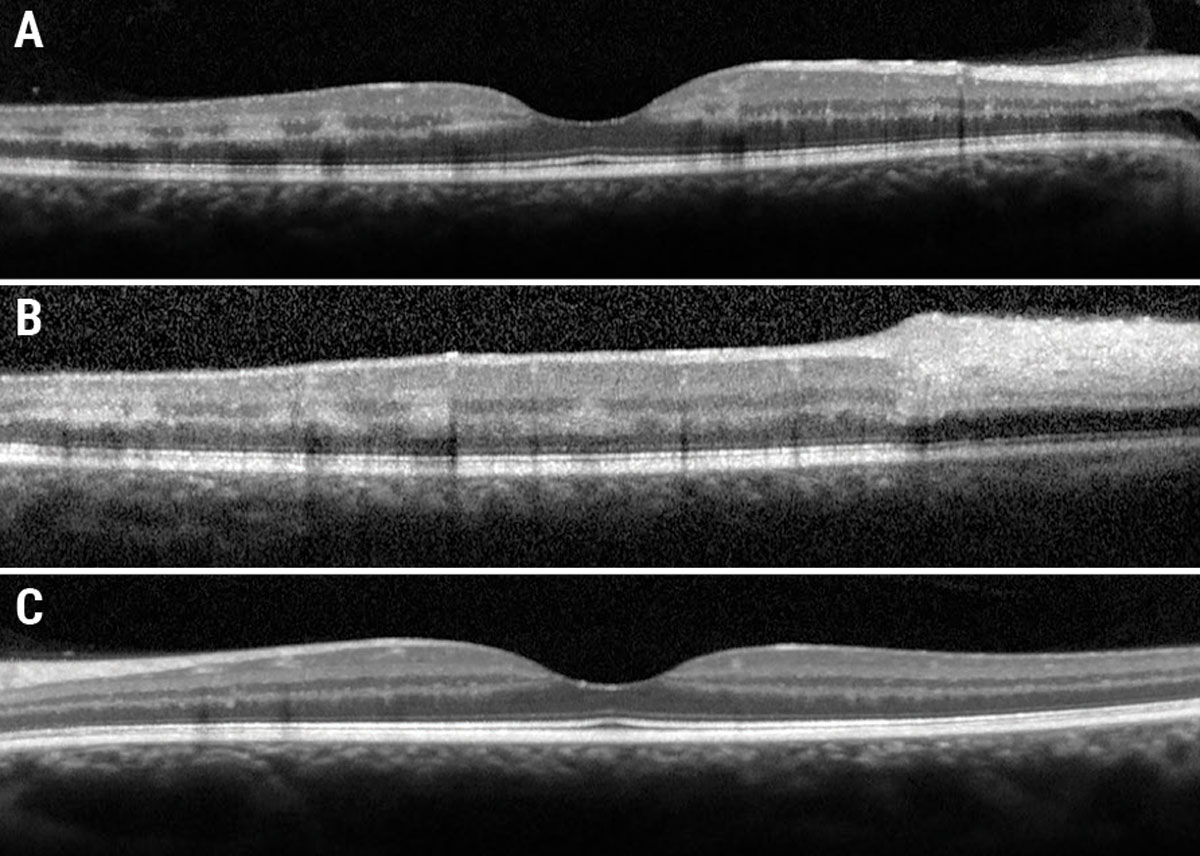
Fundus examination revealed diffuse dilated, tortuous and enlarged vessels with intraretinal and white-centered hemorrhages in all four quadrants OD (Figure 1a). There was also faint multifocal paracentral retinal whitening and confluent peripapillary retinal whitening. Of note, there was no neovascularization of the disc or elsewhere. Macular OCT showed multifocal paracentral hyper-reflectivity within the INL and OPL (Figure 2a), as well as confluent inner-retinal thickening and hyperreflectivity adjacent to the optic nerve OD (Figure 2b). Fundus exam and OCT were normal OS (Figures 1b and 2c).
Our patient was diagnosed with a combined CRVO with paracentral acute middle maculopathy (PAMM) and cilioretinal artery occlusion (CLRAO), which prompted immediate transfer to our affiliated general hospital for emergent stroke work-up. He underwent extensive bloodwork and neuroimaging, which revealed marked hyperlipoproteinemia(a) measuring 222nmol/L.
Combined CRVO/CLRAO is present in 27% to 62% of all CLRAOs. The most common risk factors for CRVO +/- CLRAOs are age greater than 50 years, hypertension, hyperlipidemia, diabetes, cigarette smoking and a positive family history of cardiovascular disease; it is rarely seen in an otherwise healthy population.1 Patients often describe a “dark spot” in their vision with typical fundus features of dilated and tortuous vessels, intraretinal hemorrhages in all quadrants, cotton wools spots and optic disc edema; retinal artery occlusion (RAO), when present, may show segmental or confluent retinal whitening.1,2
PAMM can be seen in a broader age range of patients due to variable etiologies but is more frequently found concomitantly with CRVO +/- RAO.3 Clinically, PAMM lesions appear subtly white/gray and on OCT show hyperreflective bands within the INL and OPL.3 While PAMM can be idiopathic, it warrants systemic evaluation to rule out occult etiologies.3
Lipoprotein A, or Lp(a), is a form of low-density lipoprotein involved in tissue repair and wound healing within the body.4 Lp(a) is also a known risk factor for atherosclerosis, coronary artery disease, stroke, thrombosis and aortic stenosis.4 Up to about 90% of plasma Lp(a) levels are determined by the LPA gene, making Lp(a) a non-modifiable risk factor and LPA gene expression is variable due to size polymorphism.5,6 This results in a wide range of Lp(a) levels within the population, and makes it challenging to come up with a high-risk threshold value. However, studies suggest plasma levels greater than 30mg/dL to 50mg/dL (75nmol/L to 125nmol/L) are correlated with an increased risk of cardiovascular disease.7,8 Current literature has demonstrated greater levels of Lp(a) within African populations vs. Caucasian and Hispanic populations.9
The role of Lp(a) in retinal vascular occlusion is thought to be a twofold pathogenic process.10 First, Lp(a) acts on retinal arterioles and venules by increasing vascular inflammation and has a strong affinity for the glycosaminoglycans within the arterial walls of humans.10,11 As it lodges itself within the walls, it recruits pro-inflammatory factors, which induces degeneration and scarring of the walls.11 This process leads to restricted blood flow and thrombus formation. Additionally, Lp(a) impairs the fibrinolysis process, making an individual more prone to clotting.10 The above mechanism can produce both retinal arterial and venous occlusions by way of arteriolar stenosis, thrombus formation and impaired fibrinolysis, accounting for this patient’s clinical presentation.
Lp(a) is a known independent risk factor for heart disease, even in young and otherwise healthy patients.4 While its role in retinal disease is a budding area of research, studies suggest a correlation between CRVO and hyperlipoproteinemia(a).10 This raises the question of whether Lp(a) should be included in the routine work-up for young patients with CRVO/RAO without known risk factors, as well as what to do with borderline positive results given our incomplete understanding of what constitutes a normal range.
 |
|
Fig. 2. (a) Foveal OCT OD. (b) Eccentric OCT superior to fovea. (c) Foveal OCT OS. Click image to enlarge. |
Treatment
Ophthalmic intervention depends on the presentation. Non-ischemic CRVOs without cystoid macular edema are monitored with serial dilation and macular OCT.12 Ischemic CRVOs should be followed more closely, typically once a month for the first six months with serial gonioscopy to monitor for anterior segment neovascularization (seen in about 50% of patients).12 Both neovascularization and cystoid macular edema are indications for intravitreal anti-VEGF injections.12 Systemic work-up is indicated in young patients or those without an obvious systemic risk factor. PAMM and RAOs are often managed similarly as warranting an immediate systemic evaluation to rule out concomitant cerebrovascular accident or underlying etiology requiring intervention.3,13
In the case of hyperlipoproteinemia(a), patients should establish care with a cardiologist who is likely to initiate lipid-lowering therapy. Currently, there are no drugs on the market directly targeting Lp(a). However, studies have shown reduction in Lp(a) levels with statins, aspirin (15% to 20%), PCSK9-inhibitors (30%) and nicotinic acid (38%).4 At present, the only FDA-approved therapy to directly reduce Lp(a) levels is lipoprotein apheresis which yields a 60% to 80% reduction in Lp(a) levels but is reserved for more extreme disease states.14
Our patient was started on atorvastatin 80mg and aspirin 325mg daily in the emergency department and is currently under the care of the cardiology team. Additionally, he has been followed by our retina service with resolution of PAMM lesions and no occurrence of neovascularization or cystoid macular edema, and his vision has remained stable at 20/20.
Retina Quiz Answers
1: d, 2: b, 3: a, 4: a, 5: d
Dr. Aboumourad currently practices at Bascom Palmer Eye Institute in Miami. Dr. Bergstrom graduated from the Southern College of Optometry and is completing an ocular disease residency at Bascom Palmer Eye Institute. They have no financial disclosures.
1. Grzybowski A, Elikowski W, Gaca-Wysocka M. Cardiovascular risk factors in patients with combined central retinal vein occlusion and cilioretinal artery occlusion. Medicine (Baltimore). 2018;97(1):e9255. 2. Bagheri N. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. Wolters Kluwer; 2017. 3. Mishra P, Mohanty S, Shanmugasundaram P, et al. Paracentral acute middle maculopathy as the presenting sign of ischemic cardiomyopathy. Cureus 2023;15(2):e35418. 4. Farzam K, Senthilkumaran S. Lipoprotein A. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. January 2024. 5. Maranhão RC, Carvalho PO, Strunz CC, Pileggi F. Lipoprotein (a): Structure, pathophysiology and clinical implications. Arq Bras Cardiol. 2014;103(1):76-84. 6. Enkhmaa B, Anuurad E, Berglund L. Lipoprotein (a): impact by ethnicity and environmental and medical conditions. J Lipid Res. 2016;57(7):1111-25. 7. Siekmeier R, Scharnagl H, Kostner GM, et al. Variation of Lp(a) plasma concentrations in health and disease. The Open Clinical Biochemistry Journal 2010;3:72-89. 8. Svilaas T, Klemsdal TO, Bogsrud MP, et al. High levels of lipoprotein(a) – assessment and treatment. Tidsskr Nor Laegeforen. 2022;142(1). 9. Mehta A, Jain V, Saeed A, et al. Lipoprotein(a) and ethnicities. Atherosclerosis 2022;349:42-52. 10. Paciullo F, Giannandrea D, Virgili G, et al. Role of increased lipoprotein (a) in retinal vein occlusion: a systematic review and meta-analysis. TH Open. 2021;05:e295-302. 11. Grassi P, A Salicone, Motta L, Motta M. Central retinal vein occlusion associated with high blood levels of lipoprotein (a). Saudi J Ophthalmol. 2016;30(4):250-2. 12. Central Retinal Vein Occlusion. EyeWiki. Aaoorg. October 2019. 13. Mac Grory B, Schrag M, Biousse V, et al. Management of central retinal artery occlusion: a scientific statement from the American Heart Association. Stroke. 2021;52(6):e282-94. 14. Thompson G, Parhofer KG. Current role of lipoprotein apheresis. Curr Atheroscler Rep. 2019;21:(7):26. |

