 A 70-year-old white female presented for an eye examination with a chief complaint of a painless, progressive visual blur in both eyes that had persisted for 18 months. Her last eye exam was more than four years ago. At that time, we recommended that she use reading glasses. The glasses helped for a little while, but she now believed that her prescription was not strong enough.
A 70-year-old white female presented for an eye examination with a chief complaint of a painless, progressive visual blur in both eyes that had persisted for 18 months. Her last eye exam was more than four years ago. At that time, we recommended that she use reading glasses. The glasses helped for a little while, but she now believed that her prescription was not strong enough.
Her medical history was significant for hypertension and familial polyposis adenomatous (FAP), which required partial intestinal removal.
On examination, her entering visual acuity measured 20/30 OD and 20/25 OS, with a manifest refraction of 20/20 OU. Extraocular motility testing was normal. Confrontation visual fields were full to careful finger counting OU. Her pupils were equally round and reactive, with no evidence of afferent defect. Her anterior segment examination was remarkable for 1+ nuclear sclerotic cataracts OU.
Dilated fundus examination of both eyes revealed small cups, with good rim perfusion and coloration. We noted obvious macular changes in both eyes (figures 1 and 2). Further, we documented retinal pigment epithelium (RPE) changes in her left eye (the vessels and periphery were normal, however).
Additionally, we ordered a spectral-domain optical coherence tomography (SD-OCT) scan (figures 3 and 4).
Take the Retina Quiz
1. What do the posterior poll changes represent OU?
a. Drusen.
b. Exudate.
c. Lipofuscin.
d. RPE atrophy.
2. What findings do the SD-OCT scans reveal?
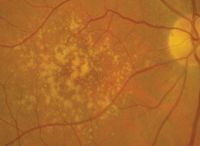
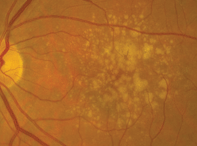
1, 2. Fundus images of our patient show obvious macular changes (OD left, OS right). What do these findings represent?
a. Drusen at the level of the RPE.
b. Geographic atrophy.
c. Occult choroidal neovascularization (CNV).
d. Nonspecific subretinal fluid.
3. What is the correct diagnosis?
a. Dry AMD.
b. Wet AMD.
c. Stargardt’s macular degeneration (SMD).
d. Fundus albipunctatus.
4. What additional testing would be most helpful for this patient?
a. Fluorescein angiogram.
b. Genetic testing.
c. Fundus autofluoresence (FAF).
d. All of the above.
5. How should this patient be managed?
a. Observation.
b. Intravitreal anti-VEGF injection.
c. Nutritional supplements that contain lutein and zeaxanthin.
d. Management decisions are contingent upon the outcome of further testing.
For answers, scroll to the bottom.
Discussion
The changes seen in our patient’s maculae represent drusen, which are almost always pathognomonic for macular degeneration. But, is the macular degeneration dry or wet? This is always one of the key questions to address when examining any patient with AMD.
Sometimes it’s fairly obvious. But, in other instances, you have to rely on clues in your examination (e.g., the presence of subretinal fluid, exudate or even hemorrhage) to determine if CNV is present. In the absence of these findings, it can be challenging and, at times, nearly impossible.
Fortunately, the advent of OCT has made it much easier to determine if the patient has converted from dry to wet AMD.
Even though our patient exhibits extensive drusen, we did not detect any of the key features indicative of CNV. Additionally, her maculae looked completely flat—so I was fairly certain that she had not converted.
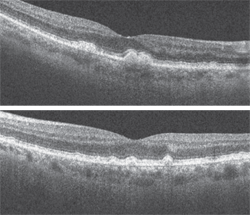
3, 4. What clinical findings are revealed on SD-OCT (OD top, OS bottom)?
Nonetheless, we obtained an SD-OCT not only to confirm our suspicions, but also to provide essential baseline information for future follow-up. The SD-OCT revealed focal elevations at the level of the RPE in both eyes, which represented the drusen. More importantly, the scan documented no subretinal fluid in either eye. Fortunately, both the clinical evaluation and ancillary testing supported a diagnosis of dry AMD.
Despite her “favorable” diagnosis, we had to address several critical questions. How should she be followed? Considering the extensive drusen formation, should she be seen more frequently than once or twice a year? And, most importantly, what was her overall risk of converting to wet AMD?
Based on the amount of drusen, we intuitively assumed that her conversion risk was reasonably high. But, to be absolutely certain, we discussed the option of genetic testing.
Macula Risk (ArcticDx) is a genetic test that can be performed in the office with a simple cheek swab. The test accounts for all the known genetic components of AMD that predispose a patient to disease progression. The test analyzes the effects of inflammation (via the complement factor proteins), oxidative damage and mitochondrial health, as well as adjusts for a history of tobacco smoking.
Following testing, Macula Risk assigns a patient to a risk category—ranging from 1 to 5. Patients in categories 4 and 5 have the highest risk of progression, with more than an 83% predictive value.1 Such individuals should be seen three to four times per year, and undergo careful fundus evaluations and retinal imaging (e.g., SD-OCT or FAF).
Interestingly, our patient specifically inquired if genetic testing was available, because her mother had suffered vision loss from AMD. Further, she suggested that a distant relative developed colon cancer as a result of FAP several years ago. At that time, her primary care physician recommended genetic testing for FAP. To her surprise, the test was positive. Additional testing revealed that she had early colon cancer, which resulted in partial removal of her large intestines. So, needless to say, our patient was a strong believer in genetic testing.
We performed Macula Risk testing on our patient, which placed her in category 4. Indeed, she was at considerable risk for AMD progression. As a result, we recommended nutritional supplementation with lutein, zeaxanthin and omega-3 fatty acid.
We instructed her to return for follow-up in four months, and will continue to monitor her at least three times per year.
1. Seddon JM, Reynolds R, Maller J, et al. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis Sci. 2009 May;50(5):2044-53.
Answers
1. a
2. a
3. a
4. d
5. d

