12th Annual Pharamaceuticals ReportCheck out the other feature articles in this month's issue: |
The mid-2000s were an exciting time in retina care. First, the FDA approved Macugen (pegaptanib, then from Eyetech, now Bausch + Lomb) for wet age-related macular degeneration (AMD) in December 2004. It showed some ability to maintain, but not improve, vision. That alone was a boon in an era when steady, progressive decline was the fate of most AMD patients. A year later, Avastin (bevacizumab, Genentech) was shown to be effective in restoring lost vision, introducing a low-cost but off-label treatment—and beating to market the same company’s branded drug Lucentis (ranibizumab, Genetech), which arrived in 2006. A third option, Eylea (aflibercept, Regeneron), was approved in 2011.
These ground-breaking therapies revolutionized retinal vascular disease management and set a new standard of care. Today, they are first-line treatments for most cases of neovascular AMD.1 All work by targeting vascular endothelial growth factor (VEGF).
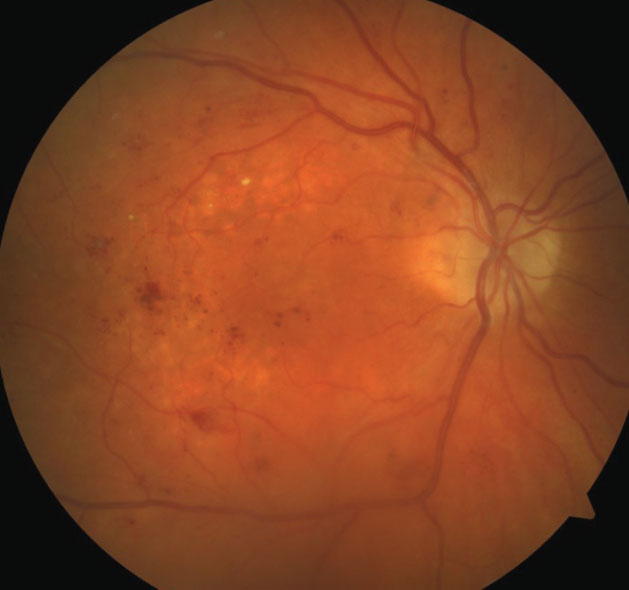 |
| Fig. 1. This fundus photo of a 71-year-old patient’s right eye shows moderate nonproliferative diabetic retinopathy with diabetic macular edema. |
Beginning in the 1970s, with confirmation in 1989, research identified VEGF as a growth factor necessary for tumor angiogenesis.2-4 VEGF also promotes vascular permeability. A 1994 study showed retinal hypoxia inducing intraocular VEGF release correlating with neovascularization in primates.5 Several human and primate studies to follow demonstrated VEGF played a direct role in the stimulation of intraocular neovascularization, and significant VEGF was found within intraocular fluid of patients with active neovascularization.6-11
This article will review both the academic literature and industry-sponsored investigations into this sight-preserving class of medication. It will also review two clinical examples of patients receiving injections as well as examine where anti-VEGF therapy stands today.
Ongoing Trials
Anti-VEGF therapy has become the standard of care for a host of intraocular vascular diseases. Eylea, Lucentis and the off-label use of Avastin are providing patients effective treatment and the ability to receive alternative treatment options during management (Table 1). As demonstrated in the CATT and IVAN trials comparing Avastin and Lucentis for wet AMD—as well as the VIEW 1 and VIEW 2 trials comparing varying dosages of Eylea and Lucentis for wet AMD—retina specialists can decide the best course of treatment based upon availability and other external factors, such as cost to the patient, without compromising quality of care.12-15
With the available anti-VEGF agents, current research is continuing to improve upon optimal treatment delivery.11-14 As of August 2018, the FDA approved a supplemental Biologics License Application for the use of Eylea every 12 weeks following one year of successful therapy on an every-four-week or every-eight-week regimen based on the Phase III outcomes of VIEW 1 and VIEW 2 clinical trials.16 This means some patients can receive injections quarterly as opposed to every eight weeks. Decreasing injection frequency may also impact long-term outcomes of wet AMD, as previous studies found more frequent doses may increase the potential for geographic atrophy (GA).12,13,17
Table 1. Currently Employed Anti-VEGF Treatment Options | ||
| Drug Name | FDA Indication | Associated Clinical Trials |
| Eylea |
|
|
| Lucentis |
|
|
| Avastin | Off-labb | CATT |
A few of the ongoing clinical trials are:
• CAN-TREAT, assessing Lucentis dosage and extended frequency for wet AMD.
• LUMINOUS, assessing five-year outcomes of safety, efficacy, treatment patterns and quality of life for wet AMD using Lucentis.
• RIVAL, comparing Lucentis and Eylea using a treat-and-extend regimen and subsequent development of GA over a two year period, as presented as interim data at the 17th EURetina Congress in Barcelona.18-20
These ongoing studies will provide results in the next one to four years and aim to give clinicians the best practice guide in managing wet AMD patients with current anti-VEGF options.
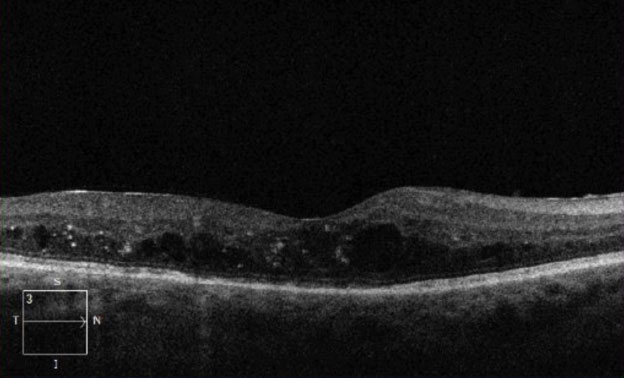 |
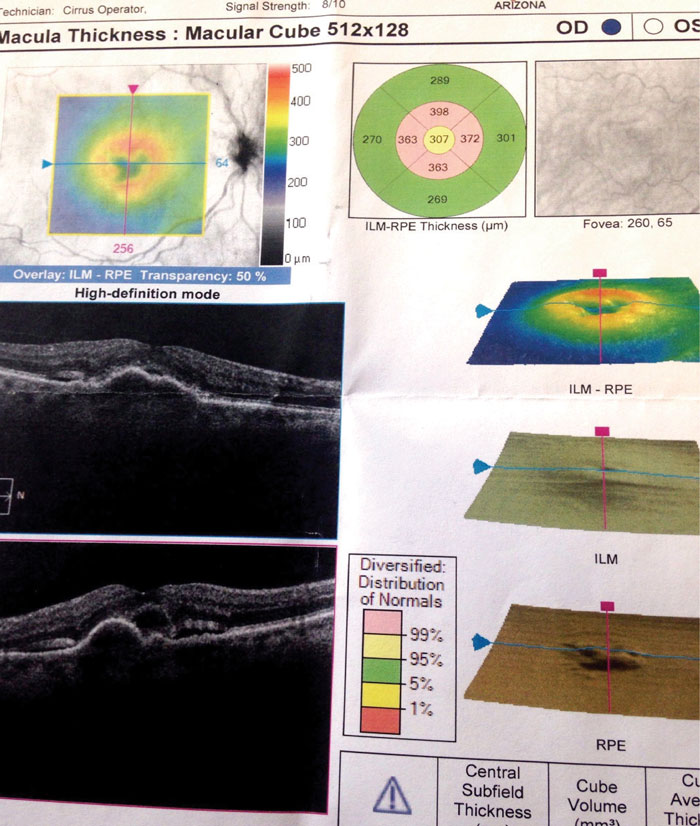
| Fig. 2. This OCT of the same eye from Figure 1 shows intraretinal fluid consistent with diabetic macular edema. |
The benefits of anti-VEGF treatment for visually significant DME and proliferative diabetic retinopathy (PDR) is clearly defined (Table 1). Recently published data from the PROTEUS study shows that Lucentis plus panretinal photocoagulation (PRP) is more beneficial for regression of neovascularization in high-risk PDR than PRP alone.20 The CLARITY noninferiority trial found that Eylea is superior in preserving acuity at one-year than standard-of-care PRP.21 Current research involving the treatment of nonproliferative diabetic retinopathy (NPDR) and asymptomatic DME with anti-VEGF therapies is looking to provide more concise clinical guidelines for patients who may commonly be under observation only. In addition to already-approved Lucentis for DR (with AMD without macular edema), Regeneron will look to obtain FDA approval for Eylea’s use in moderate to severe NPDR without macular edema. Based on preliminary results of the PANORAMA study, as well as preliminary results from DRCR.net Protocol W, Regeneron will look to expand its market options for Eylea.22,23
DRCR.net Protocol V is an ongoing trial assessing the management of DME with very good vision (20/25 or better) with observation plus deferred anti-VEGF prompt focal laser plus deferred anti-VEGF, or prompt Eylea.24
Two Anti-VEGF Uses in Clinical PracticeCase One Entrance testing yielded best-corrected distance acuities of 20/70- OD and 20/30 OS. His pupils were round and reactive to light without afferent pupillary defect in either eye. Confrontation visual fields were full to finger counting in each eye. Subjective refraction yielded no visual acuity improvement at distance with any lens in the right eye. Intraocular pressure using Goldmann tonometry yielded 13mm Hg OD and 14mm Hg OS at 09:38am. A slit lamp exam revealed mild dermatochalasis, conjunctivochalasis, and 1+ nuclear sclerosis in both eyes. Dilated fundus exam revealed 0.10 cup-to-disc ratios and good color in each eye. The retinal vascular assessment yielded mild attenuation in each eye with mild arterio-venous crossing changes. Posterior pole evaluation showed intraretinal hemorrhages throughout arcades and into macula in both eyes with a few exudates in superior macula of the right eye only (Figure 1). The peripheral retina was flat and intact in both eyes. Optical coherence tomography (OCT) was performed on the macula (Figure 2). The patient was diagnosed with moderate nonproliferative diabetic retinopathy with diabetic macular edema and referred to our retina clinic for evaluation and treatment. Visit 2: He was examined by our retinal specialist two weeks later. His best corrected visual acuity remained unchanged from the initial exam. The patient received a single injection of Eylea 2.0mg in the right eye. Visit 3: The patient was re-examined by the retinal specialist four weeks later. His best-corrected visual acuities at that exam measured 20/40 OD and 20/30 OS. A macular OCT was performed and demonstrated significant improvement in the central retinal thickness (Figure 3). At date of publication, the patient will be awaiting follow-up care.
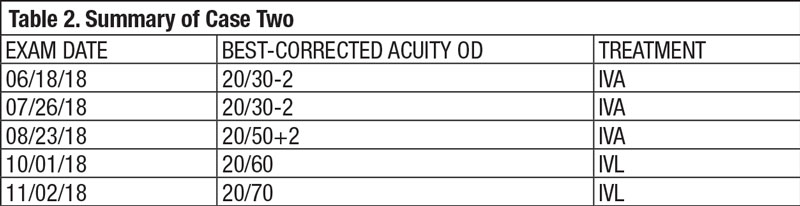
Case Two Visit 1: Two days later, she presented to the retina center with a complaint of sudden, mild blurring of her right eye. Her past ocular history was positive for visually insignificant nuclear sclerosis OU. She has a positive family history of AMD (mother). Past medical history was positive for hypothyroidism, for which she is taking levothyroxine daily. The patient had no known allergies. Entrance testing yielded a corrected distance acuity of 20/30- OD with a prescription of -0.50 DS and 20/20 OS with a prescription of -0.50 DS. Pupils were round and reactive to light without afferent pupillary defects in either eye. Confrontation fields were full to finger counting in each eye. Subjective refraction yielded no visual acuity improvement at distance with any lens in the right eye. Intraocular pressure using Goldmann tonometry yielded 15mm Hg OD and 15mm Hg OS at 9:25am. Slit lamp exam was relatively unremarkable with grade 1+ nuclear sclerosis in both eyes. Dilated fundus exam revealed 0.10 cup-to-disc ratios and good color in each eye. Retinal vascular assessment was unremarkable. Posterior pole evaluation showed a pigment epithelial detachment (PED) of the right macula with subretinal fluid, and mild drusen in both maculae. The peripheral retina was flat and intact in both eyes. OCT was performed on the macula (Figure 4). The patient was diagnosed with exudative AMD in the right eye with active choroidal neovascularization and moderate dry AMD in the left. Fluorescein angiography was not performed to confirm this diagnosis. The retina specialist elected to begin treatment at that exam with intravitreal Avastin (IVA). The patient was to follow up in four weeks with a different retinal group upon returning home. She was also instructed to begin one AREDS2 tablet, twice a day. Summary of Visits 2-6: Upon her return home, the patient was examined four weeks after her initial IVA administration (Table 2). OCT change analysis between Visit 3 and Visit 5 (Figure 5) and OCT for Visit 6 (Figure 6) are provided. Despite diligent follow up care, the response to IVA and intravitreal Lucentis (IVL) was not ideal. At time of publication, the patient will return to clinic to receive intravitreal Eylea (IVE). |
The Pipeline
The future of anti-VEGF treatment for retinal vascular conditions appears promising. In addition to ongoing studies involving optimization of current FDA-approved therapies, several new options are promising as either stand-alone options or adjunctive therapies to existing anti-VEGFs.
• Brolucizumab (Novartis). This humanized, single-chain antibody fragment inhibitor of VEGF with a molecular weight approximately four and a half times smaller than Eylea and two times smaller than Lucentis allows for greater molar concentration per injection and possibly will offer greater retinal tissue penetration and extended duration of treatment per injection.
Phase III HAWK and HARRIER trials compared Eylea 2mg every eight weeks with brolucizumab 3mg and 6mg every 12 weeks following three monthly loading doses for wet AMD.25,26 The study demonstrated non-inferiority between the two medications. Brolucizumab showed significantly reduced disease activity at week 16, superior central thickness improvements at week 16 and week 48, significantly fewer patients with intraretinal or subretinal fluid and overall safety compared with Eylea 2mg. Data reported at the American Academy of Ophthalmology 2018 meeting reaffirmed that, at 96 weeks, patients had improved retinal fluid, central retinal thickness and visual gain with brolucizumab 6mg vs. Eylea 2mg.
• Abicipar (Allergan). This anti-VEGF-A designed ankyrin repeat protein (DARPin) is highly specific, has high-affinity target binding proteins is being developed by Molecular Partners and Allergan. DARPins are recombinant proteins genetically engineered to bind to a specific protein, making them more potent, amenable to a better shelf life and highly soluble, which means they can be easily mixed with other agents for treatment. Data from the Phase II PALM study indicate abicipar injected every eight or 12 weeks in patients with DME fared as well as Lucentis 2mg injected every month.27 Initial Phase III results of the SEQUOIA and CEDAR clinical trials comparing every four-week dosing of Lucentis to every 12-week and every eight-week dosing of abicipar for wet AMD demonstrated noninferiority between the two medications with a primary endpoint of proportion of treated patients with a stable acuity at 52 weeks.27-29 These trials have a protocol assessing the primary endpoint out to 96 weeks and are ongoing at the time of publication.
• OPT-302 (Opthea). This VEGFR-3 molecule blocks the activity of VEGF-C and VEGF-D. Currently in Phase II clinical trials, researchers are exploring OPT-302 0.5mg and 2.0mg as a combination therapy with Lucentis 0.5mg in wet AMD patients. Early results appear promising for OPT-302 as an adjunctive therapy in patients responding poorly to VEGF-A therapy alone.30
 |
| Fig. 3. Progression analysis on OCT of the right eye of the patient in Case One, four weeks after initial intravitreal injection of anti-VEGF medication. |
• Conbercept (Chengdu Kanghong Biotech). This recombinant fusion protein that binds all VEGF-receptor isoforms and PIGF is currently marketed in China for wet AMD. Conbercept received Phase III clinical trial fast-track in the United States under the PANDA 1 trial comparing conbercept 0.5mg (every eight weeks) and 1.0mg (every 12 weeks) vs. Eylea 2.0mg (every eight weeks) after three total monthly loading doses.31 Although clinical trials are ongoing for conbercept, research shows it is promising, with greater VEGF and PIGF binding affinity than Lucentis 0.5mg.32,33 Conbercept, under the BRAVE trial, is also being assessed in Phase III clinical trials for macular edema associated with branch retinal vein occlusion.34 Data collection is ongoing for this study and is comparing conbercept to sham injections.
• Ziv-aflibercept (Sanofi-Aventis/Regeneron). A recombinant fusion protein with similar actions on VEGF isoforms and PIGF as Eylea, ziv-aflibercept is currently FDA-approved for metastatic colorectal cancer treatment. Previous studies looked at its potential toxicity to the retinal tissue, given its osmolarity, but these reports found no significant complications relative to certain levels of concentration.35,36 Current FDA clinical trials, such as ZALTRAP, aim to provide greater insight into toxicity and overall efficacy in several retinal conditions, including wet AMD, DME and macular edema due to retinal vein occlusion. Estimated study completion for ZALTRAP is December 2019.37 Another ongoing FDA trial, ZEBRA, will compare ziv-aflibercept 1.25mg every month vs. Eylea, Lucentis and Avastin every five to 12 weeks in patients with wet AMD to assess the overall best-corrected visual acuity outcomes.38 Data from ZEBRA may not be available until 2020 based on estimated study completion date.
 |
| Fig. 4. This OCT of the Case Two patient’s right eye at her first visit shows PED and associated subretinal and intraretinal fluid. |
• Faricimab (Genetech). This is the first bispecific antibody designed specifically for intravitreal use to simultaneously bind angiopoietin-2 (Ang-2) and VEGF-A with high potency and specificity. The Phase II STAIRWAY study demonstrates that faricimab 6.0mg every 16 weeks or every 12 weeks is comparable with ranibizumab 0.5mg every four weeks in overall improvement of acuity and central retinal thickness after a 52-week follow-up.39 The safety profiles of the two drugs are comparable, too.39
Two Phase III studies—RHINE and YOSEMITE-—are designed to investigate the efficacy and safety of faricimab compared with aflibercept in patients with DME.40,41 Additionally, in response to the STAIRWAY study, a global Phase III study for faricimab in wet AMD will commence in 2019.
• Port delivery system (PDS) with ranibizumab (Genentech). This refillable, sustained-delivery system is implanted into the globe to deliver varying dosages of Lucentis. In the Phase II LADDER study, doses of 10mg/mL, 40mg/mL, 100mg/mL ranibizumab were placed in the port-delivery system for patients with wet AMD.42 Outcomes show the medication refill was not needed for up to six months or longer and best-corrected visual acuity for the 100mg/mL dose was comparable with monthly Lucentis 0.5mg injections. The Phase III ARCHWAY trial will assess long-term acuity in patients with the PDS refilled every 24 weeks vs. monthly Lucentis 0.5mg.43
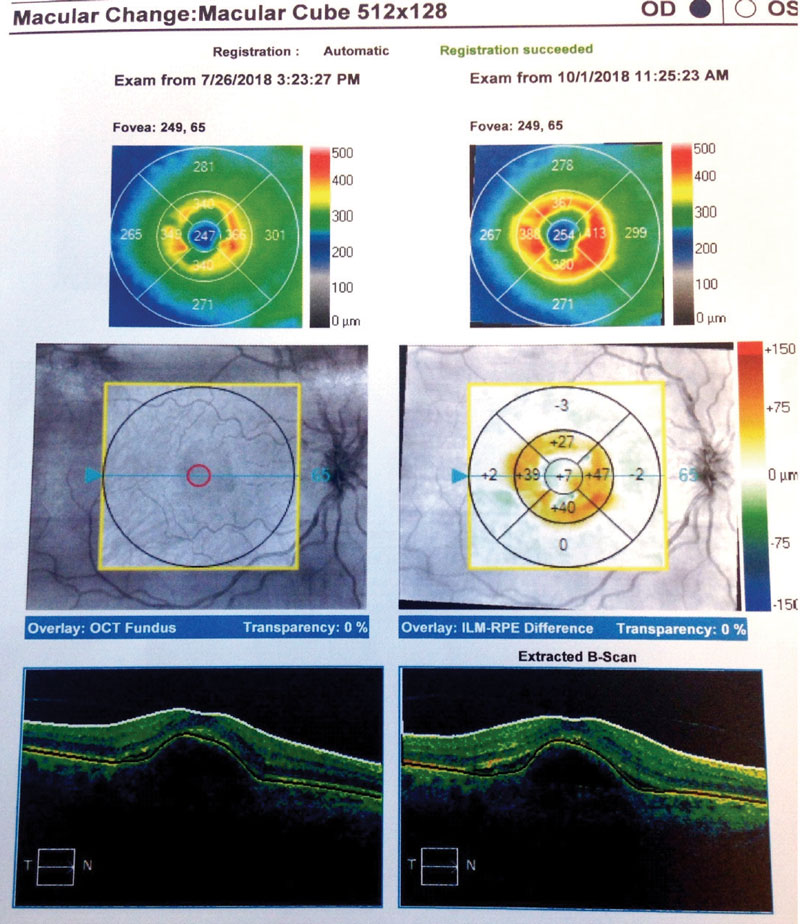 |
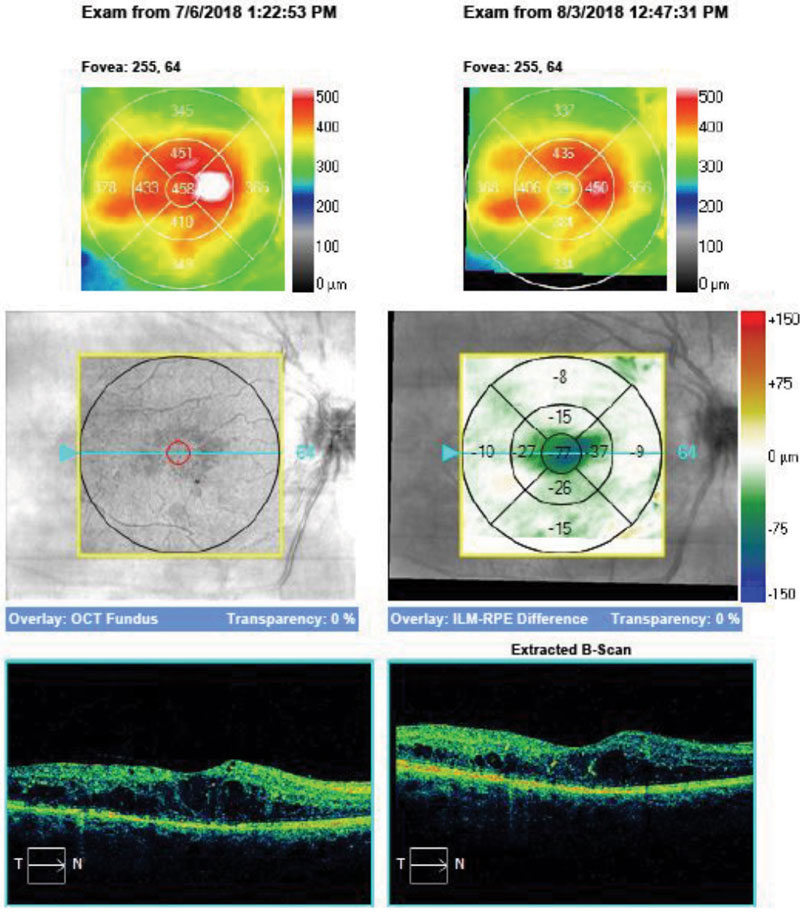
| Fig. 5. Here, our Case Two patient’s change analysis between visits three and five are displayed. You can see PED and associated intraretinal and subretinal fluid. Note the worsening of overall thickness. |
• Biosimiliars. These biological products are highly similar to, and have no clinically meaningful differences from, existing FDA-approved reference products. Unlike generics, whose active ingredients are the exact same as the approved referenced drug, biosimiliars must demonstrate that they are highly similar to the reference product, except for minor differences in clinically inactive components, and that they demonstrate no clinically meaningful difference in safety or efficacy.43 Currently biosimiliar products used outside of the United States are under Phase III investigation and demonstrate noninferiority to Lucentis.44
• RGX-314 (RegenXBio). This formulation is being developed and studied as a one-time subretinal gene therapy treatment for wet AMD. It includes a recombinant adeno-associated virus gene therapy vector that carries a coding sequence for a soluble anti-VEGF protein. RGX-314 The expressed protein is designed to neutralize VEGF activity, modifying the pathway for formation of new leaky blood vessels and retinal fluid accumulation. Patients are currently being enrolled in Phase I/IIa, open-label, multiple-cohort, dose-escalation study designed to evaluate the safety and tolerability of RGX-314 gene therapy in subjects with previously treated wet AMD.45
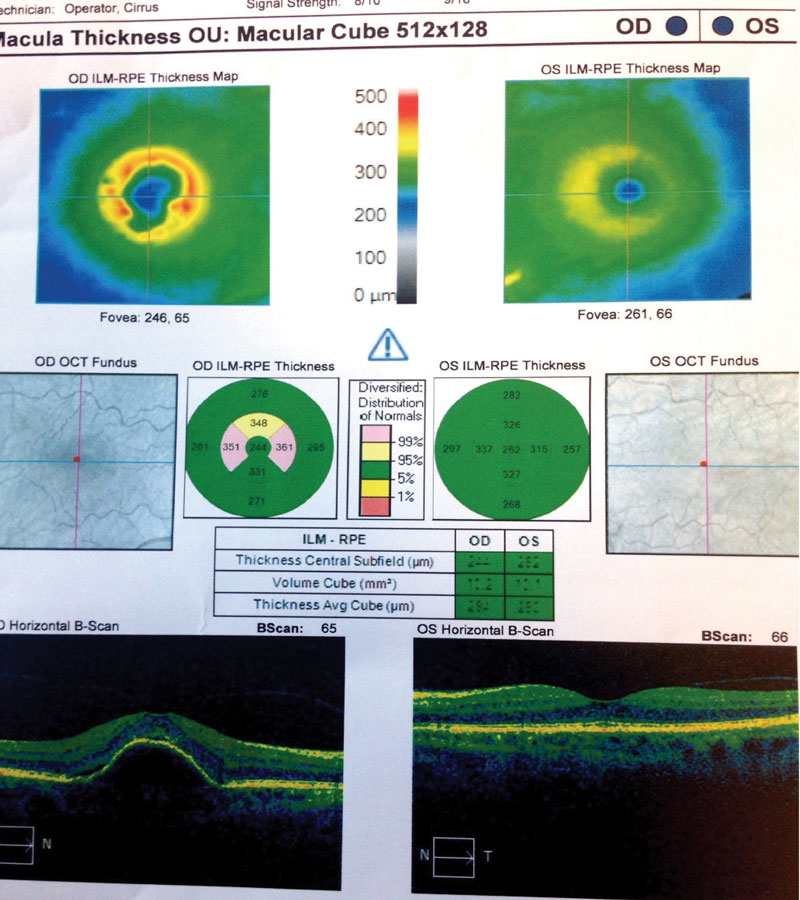 |
| Fig. 6. These OCT images show our Case Two patient at her sixth visit. You can see persistent PED with minimal intraretinal fluid in the right eye. Additionally, her left eye shows minimal RPE changes from drusen and no fluid. |
Anti-VEGF therapy has developed into the new standard of care for patients with retinal vascular disease. The advent of these medications has brought about the ability to restore and maintain vision. The future of retinal vascular care with anti-VEGF agents now depends on the ability to use existing products more effectively and efficiently, or to build upon their successes by creating next-generation drugs that address shortcomings of the initial wave. Additionally, the market is looking to provide clinicians with new options to manage these myriad conditions with optimal intraocular drug delivery and efficacy without compromising the patient’s vision, ocular health, time or finances.
Dr. Kress is an optometrist at the Cincinnati Veteran’s Administration Medical Center.
| 1. Vedula S, Krzystolik M. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for neovascular age-related macular degeneration. Cochrane Database Syst Rev 2008;2. 2. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6. 3. Leung D, Cachianes G, Kuang W, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306-9. 4. Keck P, Hauser S, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246(4935):1309-12. 5. Miller J, Adamis A, Shima D, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994;145(3):574-84. 6. Aiello L, Avery R, Arrigg P, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480-7. 7. Adamis A, Shima D, Tolentino M, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996;114(1):66-71. 8. Aiello L, Pierce E, Foley E, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92(23):10457-61. 9. Robinson G, Pierce E, Rook S, Foley E, et al. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci USA. 1996;93(10):4851-6. 10. Ozaki H, Seo M, Ozaki K, et al. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000;156(2):697-707. 11. Tolentino M, Miller J, Gragoudas E, et al. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol. 1996;114(8):964-70. 12. Group C, Martin D, Maguire M, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-908. 13. Chakravarthy U, Harding S, Rogers C, IVAN Study Investigators. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258-67. 14. Heier J, Brown D, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-48. 15. Schmidt-Erfurth U, Kaiser P, Korobelnik J, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmol. 2014;121(1):193-201. 16. FDA approves Eylea 12-week dosing for wet AMD. Ocular Surgery News. www.healio.com/ophthalmology/retina-vitreous/news/online/%7Bdbbb1669-4860-4fec-850e-5c84e2ea59fc%7D/fda-approves-eylea-12-week-dosing-for-wet-amd. August 17, 2018. Accessed February 11, 2019. 17. Rofagha S, Bhisitkul R, Boyer D, et al. SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013 Nov;120(11):2292-9. 18. Kertes P. Canadian treat and extend analysis trial with ranibizumab in patients with neovascular AMD: Interim analysis of the CANTREAT study. Presented at: EURETINA; September 7-10, 2017; Barcelona, Spain. 19. Brand C. One-year outcomes with ranibizumab in treatment naïve patients with neovascular age-related macular degeneration: An interim analysis from the LUMINOUS study. Presented at: ARVO; May 1-5, 2016; Seattle, Washington. 20. Figueira J, Fletcher E, Massin P, et al. EVICR.net Study Group. Ranibizumab plus panretinal photocoagulation versus panretinal photocoagulation alone for high-risk proliferative diabetic retinopathy (PROTEUS Study). Ophthalmology. 2018 May;125(5):691-700. 21. Sivaprasad S, Prevost A, Vasconcelos J, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicenter, single-blinded, randomized, controlled, phase 2b, non-inferiority trial. Lancet, 2017;389(10085):2193-203. 22. Study of the efficacy and safety of intravitreal (IVT) aflibercept for the improvement of moderately severe to severe nonproliferative diabetic retinopathy (NPDR) (PANORAMA) – Full Text View. NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT02718326. May 22, 2018. Accessed September 27, 2018. 23. Anti-VEGF treatment for prevention of PDR/DME – Full Text View. NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT02634333. August 24, 2018. Accessed September 27, 2018 24. Treatment for CI-DME in eyes with very good va study (Protocol V). NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT01909791. February 28, 2018. Accessed September 27, 2018. 25. Efficacy and safety of RTH258 versus aflibercept. clinicaltrials.gov/ct2/show/NCT02307682. NIH-Clinical Trials. May 10, 2018. Accessed September 28, 2018. 26. Efficacy and safety of RTH258 versus aflibercept - Study 2. NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT02434328. March 30, 2018. Accessed September 28, 2018. 27. Hassan T. A multi-center double-masked, phase 2 clinical trial evaluating abicipar pegol for diabetic macular edema. Paper presented at American Academy of Ophthalmology Annual Meeting; October 4-18, 2016, Chicago, IL 28. Safety and efficacy of abicipar pegol in patients with neovascular age-related macular degeneration. NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT02462486. May 1, 2018. Accessed October 3, 2018 29. A safety and efficacy study of abicipar pegol in patients with neovascular age-related macular degeneration (CDER). NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT02462928. May 1, 2018. Accessed October 3, 2018 30. A dose ranging study of OPT-302 with ranibizumab in neovascular (wet) AMD. NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT03345082. November 28, 2018. Accessed February 14, 2019. 31. Efficacy and Safety trial of conbercept intravitreal injection for neovascular age-related macular degeneration (PANDA-1). NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT03577899. October 10, 2018. Accessed February 14, 2019. 32. Ming Z, Zhang J, Mi Y, et al. A phase 1 study of kh902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology. 2010;118(4):672-8. 33. Zhao JL, Liang SQ, Fu W, et al. The lim domain protein fhl1c interacts with tight junction protein zo-1 contributing to the epithelial–mesenchymal transition (emt) of a breast adenocarcinoma cell line. Gene. 2014;542(2):182-9. 34. conbercept ophthalmic injection for patients with macular edema caused by branch retinal vein occlusion (BRAVE). NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT03108352. September 21, 2018. Accessed October 3, 2018 35. Mansour A, Al-Ghadban S, Yunis M, El-Sabban M. Ziv-aflibercept in macular disease. Br J Ophthalmol. 2015;99(8):1055-9. 36. Malik D, Tarek M, Caceres del Carpio J, et al. Safety profiles of anti-VEGF drugs: bevacizumab, ranibizumab, aflibercept and ziv-aflibercept on human retinal pigment epithelium cells in culture. Br J Ophthalmol. 2014;98(Suppl 1): i11–6. 37. Ziv-aflibercept in Ocular Disease Requiring Anti-VEGF Injection (Zaltrap). NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT02486484. August 20, 2018. Accessed October 5, 2018. 38. Ziv-aflibercept efficacy in better regulating AMD (ZEBRA). NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT03423823. February 6, 2018. Accessed October 5, 2018 39. study to evaluate faricimab (RO6867461; RG7716) for extended durability in the treatment of neovascular age related macular degeneration (nAMD) (STAIRWAY). NIM-Clinical Trials. clinicaltrials.gov/ct2/show/NCT03038880. January 15, 2019. Accessed February 14, 2019. 40. A Study to evaluate the efficacy and safety of faricimab (RO6867461) in participants with diabetic macular edema (RHINE). NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT03622593. February 11, 2019. Accessed February 14, 2019. 41. A study to evaluate the efficacy and safety of faricimab (RO6867461) in participants with diabetic macular edema (YOSEMITE). NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT03622580. February 11, 2019. Accessed February 14, 2019. 42. Study of the efficacy and safety of the ranibizumab port delivery system (RPDS) for sustained delivery of ranibizumab in participants with subfoveal neovascular age-related macular degeneration (AMD) (LADDER). NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT02510794. December 3, 2018. Accessed October 5, 2018 43. A Phase III Study to Evaluate the Port Delivery System Implant with Ranibizumab Compared With Monthly Ranibizumab Injections in Participants With Wet Age-Related Macular Degeneration (Archway) NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT03677934. February 11, 2019. Accessed February 14, 2019. 44. Biosimilar and Interchangeable Products. U.S. Food and Drug Administration. www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars. September 6, 2018. Accessed February 14, 2019 45. RGX-314 Gene Therapy for Neovascular AMD Trial. NIH-Clinical Trials. clinicaltrials.gov/ct2/show/NCT03066258. February 11, 2019. Accessed February 14, 2019. |