
 A 79-year-old white male presented as a new patient with a complaint of slightly decreased vision OS at both distance and near. The vision loss had been gradual for several months, he reported, and was now beginning to affect his quality of life. He said he was having difficulty driving and reading because the decreased acuity was occurring in his “better eye.”
A 79-year-old white male presented as a new patient with a complaint of slightly decreased vision OS at both distance and near. The vision loss had been gradual for several months, he reported, and was now beginning to affect his quality of life. He said he was having difficulty driving and reading because the decreased acuity was occurring in his “better eye.”
His medical history included coronary artery bypass graft (CABG) surgery 12 years earlier, and he reported moderate vision loss in his right eye (his “worse eye”) immediately following surgery. The vision OD has remained reduced since that time and he had apparently adapted well with his left eye, which maintained relatively good vision until recently. He also reported that two years prior to the vision loss in the right eye, he underwent bilateral cataract surgery. Immediately after the cataract surgery, his vision OU was quite good.
Current medications included Diovan (valsartan, Novartis), Crestor (rosuvastatin, AstraZeneca), Pacerone (amiodarone, Upsher-Smith), Plavix (clopidogrel, Bristol-Myers Squibb) and low-dose (81mg) aspirin. He reported no allergies to medications.
Diagnostic Data
Entering visual acuity was 20/200 OD and 20/70 OS. The right pupil showed a 2+ afferent defect. Extraocular motilities were full in all positions of gaze. Confrontation fields were markedly reduced in the right eye with peripheral constriction as well as what appeared to be pericentral defects. That of the left eye was limited by mild dermatochalasis superiorly. Best-corrected visual acuity was 20/80- OD and 20/50+ OS.
Slit lamp examination revealed bilateral corneal arcus and mild vortex epitheliopathy. There was mild sectoral iris atrophy OD where the posterior chamber IOL was in contact with the posterior surface of the iris. Both IOLs appeared to be well centered; the right capsule was opened, while the left showed moderate opacification on the visual axis. The anterior chambers were deep and quiet.
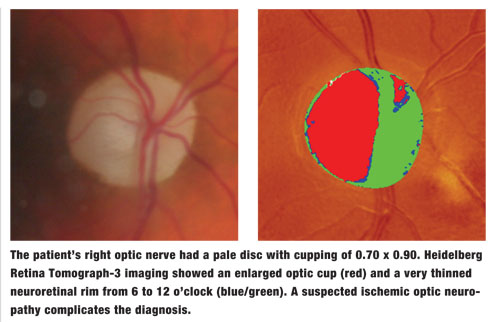
Applanation tonometry measured 24mm Hg OD and 17mm Hg OS at 10:05 AM. Upon dilation, posterior vitreous detachments were noted in both eyes. The right optic nerve had a pale disc with cupping of 0.70 x 0.90. The neuroretinal rim OD was significantly eroded from 6 to 12 o’clock; the thickest sector was nasal, and the remaining neuroretinal rim in that eye was pale and atrophic. The left optic nerve had a well-perfused neuroretinal rim with a cup-to-disc ratio of 0.45 x 0.45.
Macular evaluations in both eyes showed age-consistent retinal pigment epithelium mottling with minimal drusen outside the foveal avascular zone and no evidence of subretinal neovascular activity. Retinal vascular examinations were consistent with moderate arteriolarsclerotic retinopathy OU, with attenuated retinal vasculature OD that corresponded with the optic atrophy and vision loss. His peripheral retinal examinations were unremarkable.
Given this was a new patient with signs of both optic atrophy and neuroretinal rim thinning OD, I carefully reviewed his history with particular consideration of his complaints of vision loss OD. He was certain that, post-cataract surgery OU, his vision was quite good in both eyes, but that immediately following the CABG surgery, he noticed a significant decrease in vision in the right eye. He reported having had a “stroke” in that eye. I carefully asked him if his vision worsened gradually in any way, trying to determine if the neuroretinal rim loss occurred after what was apparently an intraoperative/postoperative ischemic optic neuropathy. He reported that it was hard to determine because he relied more on his left eye from that point on.
Treatment and Management
In the interest of improving the patient’s vision, we scheduled him for a YAG capsulotomy OS, as opacification of that posterior capsule was the etiology of his presenting visual complaints. The YAG was completed the following week. Post-YAG evaluation demonstrated no IOP rise, a quiet anterior chamber, no retinal problems and BCVA of 20/30+2 OS.
However, we were still left with the confounding issue related to his right optic nerve and decreased vision in that eye. Accordingly, I asked the patient to return for essentially a glaucoma workup, with perimetry aimed at differentiating ischemic optic neuropathy from glaucomatous field loss.
At this workup, IOP was 25mm Hg OD and 19mm Hg OS. Pachymetry measured central corneal thicknesses of 529µm OD and 540µm OS. Threshold visual fields were unremarkable in the left eye, but the right eye demonstrated superior cecocentral scotoma consistent with anterior ischemic optic neuropathy, as well as overlying arcuate field defects and nasal step inferiorly consistent with glaucomatous optic neuropathy.
Gonioscopy demonstrated 4+ open angles with slightly more trabecular pigmentation OD than OS, and no other angle abnormalities. Tomography of the optic nerves confirmed significant neuroretinal rim loss OD and a relatively robust neuroretinal rim OS. OCT imaging of the left retinal nerve fiber layer (RNFL) was normal while the right RNFL showed 360° thinning.
Discussion
This patient presented with profound vision loss OD attributed to an ischemic event tied to cardiovascular surgery, which was supported by the field loss and optic nerve pallor. A quick evaluation of the optic nerve would lead one to completely equate the vision loss OD with the nerve pallor resulting from some type of ischemic event at the time of his bypass surgery.
However, he also presented with an extremely thinned and pale neuroretinal rim. Even in the presence of a pale, atrophic disc, one must closely evaluate the remaining neuroretinal rim tissue for signs of its overall health—in this case, optic neuropathy.
Optic neuropathies are generally divided into glaucomatous and non-glaucomatous types.
Glaucomatous optic neuropathies tend to damage the optic nerves in characteristic fashion, with progressive thinning and loss of neuroretinal rim volume and mass, resulting in a progressive increase in the cup-to-disc ratio, loss of perioptic RNFL (especially in the inferior and superotemporal regions) and ganglion cell loss extending into the perifoveal regions.
Non-glaucomatous optic neuropathies (AAION, NAAION, PION, to name a few) don’t tend to cause loss but rather death of ganglion cells at the optic nerve, and those ganglion cells that do survive are pale and atrophic.
In our patient, the loss of ganglion cells, the subsequent thinning of the neuroretinal rim and the increased cupping are more indicative of glaucomatous optic neuropathy than ischemic optic neuropathy.
So can a patient have both diseases—glaucoma and ischemic optic neuropathy—at the same time? Of course, and this patient is a prime example. Moreover, something needs to be done to prevent further compromise to his right optic nerve. Accordingly, I prescribed Travatan Z (travoprost, Alcon) HS, OD only.
My speculation is that the patient suffered an acute ischemic event, resulting in death and pallor of ganglion cells OD and resultant vision loss, and also had glaucoma, which, by the time I saw him, had progressed enough to erode further the fragile, ischemic ganglion cells in the typical way that glaucomatous optic neuropathy does—namely, loss of ganglion cells. Could the ischemic event and resultant pallor make the remaining ganglion cells more susceptible to other types of damage, such as glaucomatous damage? Certainly, and that’s what I think happened in this case.
Whether the glaucoma existed undetected before or after the ischemic event is a moot point. The remaining pale, compromised ganglion cells were damaged in a fashion consistent with glaucoma. Medical intervention is essential in preventing further ganglion cell loss. n

