The vision loss that may accompany wet age-related macular degeneration is vexing not only owing to the toll it exacts on the patient’s quality of life but also because the disease often resists an eye doctor’s best efforts to prevent such an outcome. This is tremendously frustrating to any clinician who manages these patients. While the concern of eye doctors is to act in their patients’ best interests, outcomes may not always match intentions. Clinicians are then left to accept a rather dismal course of events, with patients undergoing chronic therapy and not necessarily showing notable improvement.
With the introduction of the injectable anti-vascular endothelial growth factor (VEGF) agent Avastin (bevacizumab, Genentech) in 2004 as an ingenious off-label use and then the FDA approval of Lucentis (ranibizumab, Genentech) in 2006, doctors finally had treatments with which to arrest the advance of macular degeneration in some patients, and to improve vision to by potentially as much as three lines of Snellen acuity. The introduction of Eylea (aflibercept, Regeneron) in 2011 brought to the scene a third medication of this kind. Its duration of activity is said to be longer than that of its counterparts, thus perhaps allowing for greater intervals between injections, although clinical response can vary. “It seems to do a much better job of ‘drying’ the area, with less frequent injections,” says Paul M. Karpecki, OD.
“Currently, the best available treatment for wet AMD is intravitreal anti-VEGF, which has been a tremendous boon for a disease where there was previously little hope of vision stabilization, let alone any sort of recovery,” says Elyse L. Chaglasian, OD, an associate professor at the Illinois College of Optometry in Chicago.
In some patients with wet AMD, however, intravitreal anti-VEGF injections have resulted in little more than marginal visual improvement. Their limited clinical efficacy is not the only problem. The injections themselves may not be painful—retina specialists of course use topical lidocaine to numb the site—but no one looks forward to having a needle stuck in their eye. Returning for monthly or otherwise periodic injections is inconvenient not only for the patient but also in many cases for the caregiver who must drive the patient to the clinic. The cost to insurers, and by extension to society—the on-label drugs cost about $2,000 per injection—cannot be gainsaid. Fortunately, most patients can participate in copay assistance programs, often making the cost less than that of off-label options, says Dr. Karpecki.
All of which leaves eye doctors in something of a quandary. “The current system of inject, inject, inject is really not sustainable,” says Steven Ferrucci, OD, of the US Department of Veteran Affairs in North Hills, Calif., and a professor at the Southern California College of Optometry at Marshall B. Ketchum University in Los Angeles. “So, we have to find other ways—whether it’s longer-acting injections, or drops, or an oral agent that works as an adjunct to allow for fewer injections. I don’t know what the answer is, but we do need to do something.”
Anti-VEGF injections, while demonstrably beneficial, suffer from some fundamental drawbacks. A care protocol revolutionized a decade ago is already in need of another leap forward.
|
|
AMD Drug Delivery Developments
Exploring the topical route, researchers wish to develop means to penetrate the eye’s anatomical barriers so that an agent can arrive at the target tissue in adequately potent therapeutic concentrations. Using animal models, researchers at University College of London were able to create formulations of tiny nanoparticles loaded with Avastin to deliver significant topical concentrations of the drug to the macula. This is especially notable in that Avastin and Lucentis were thought to consist of molecules too large to penetrate the cornea as an eye drop. Lucentis-maker Genentech has reported progress in using a device known as a port delivery system aimed at reducing the number of injections in wet AMD patients. The tiny capsule is implanted in the eye above the iris. In subsequent visits, the doctor can then refill the capsule with an anti-VEGF agent such as Lucentis. Early research showed that the delivery system can provide four months of therapy before the next refill. Another new sustained-release drug-delivery device is under development at pSivida, whose Durasert technology is designed to deliver Avastin through an injection of a powdery, bioerodable and porous silicon capsule. The drug is released as the silicon erodes. The Durasert is also designed to treat diabetic macular edema, posterior uveitis and glaucoma. Meantime, Ophthotech has completed a Phase II study of Fovista, an anti-platelet-derived growth factor (PDGF) agent designed for use with anti-VEGF drugs. The study found that patients treated with a combination of Fovista and Lucentis gained an average of 10.6 letters vs. 6.5 letters for those receiving Lucentis monotherapy. Phase III studies are underway. Fovista prevents PDGF from binding to receptors on pericytes, the external cells of new blood vessels, thereby stripping off these cells. This leaves the endothelial cells unprotected and thus more readily vulnerable to the effects of anti-VEGF agents. This combined activity would enhance the treatment’s efficacy and thereby reduce the number of injections needed to bring about a desirable therapeutic effect. |
This is why ongoing development of the topical anti-angiogenic agent squalamine lactate 0.2% (Ohr Pharmaceutical) is exciting to the eye doctors who follow its progress. The prospect of an eye drop that patients may instill daily to inhibit neovascular growth in wet AMD and forestall the consequent vision loss is one eye doctors look to as a promising potential means to counteract the frustration that occurs with present treatment options.
Swimming With Sharks
Squalamine is a small-molecule anti-angiogenic aminosterol agent that inhibits multiple growth factors and pathways responsible for angiogenesis. These include not only VEGF, but also platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF). It binds to and “chaperones” the messenger protein calmodulin to prevent growth factor signaling.
Discovered in the tissues of the dogfish shark (Squalus acanthias), squalamine was found early on to have potent broad-spectrum antimicrobial activity, which may explain the predator’s hardy resistance to infection. Besides its use in wet AMD, squalamine is currently being investigated for the treatment of diabetic retinopathy.
Its numerous avenues of anti-angiogenic activity give reason to suspect that squalamine may yield clinically salutary effects in a synergistic mechanism with anti-VEGF injectable medications. “The drug addresses multiple endogenous protein growth factors of angiogenesis,” says Joseph J. Pizzimenti, OD, an attending optometric physician at the Nova Southeastern University College of Optometry in Ft. Lauderdale. “Attacking choroidal neovascularization from several pathways represents the best chance of minimizing vision loss.”
Which sounds good in theory, but how much of the topical drop actually reaches the target tissue in the posterior segment? There’s no way to know other than what might be inferred from clinical outcomes. But the molecule’s mechanism of drug delivery suggests it may achieve therapeutic levels.
Dr. Chaglasian charts the drop’s itinerary on its journey to the macula. Squalamine first enters the conjunctiva and anterior sclera, and begins penetrating the corneal epithelium. “A mucoadhesive agent increases corneal residence time so that the drug diffuses slowly to the posterior sclera, resulting in delivery of sustained concentrations of squalamine via retardation of loss of the drug through nasolacrimal duct drainage.” Viscosity-enhancing properties soothe the ocular surface, a penetration-enhancing agent allows for greater diffusion into the corneal epithelium and a stabilizing agent acts as an antioxidant and can thwart the chemical degradation of the formulation, she explains. “Buffering agents allow for the drug to be at a near-neutral pH, compatible with ocular administration,” Dr. Chaglasian says. “The tonicity modifier in the formulation produces the appropriate osmolality of the ophthalmic formulation.”
Trials and Ministrations
Even the most glorious drug delivery mechanism is worth little if the drug being delivered arrives at its target with little to say for itself. The results from the recently completed Phase II FDA trials show visual improvement at nine months in wet AMD patients receiving a combination of Lucentis injections plus squalamine vs. monotherapy with just Lucentis.1
|
|
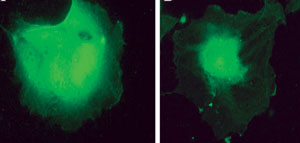
| At left is an untreated endothelial cell. At right is a cell treated with squalamine—the drug binds to the messenger protein calmodulin to prevent growth factor signaling. Photo: Ohr Pharmaceutical |
Ohr’s interim data show that 48.3% of those receiving the combination therapy had gains of three lines or more of best-corrected visual acuity on the ETDRS chart, vs. 21.2% treated solely with Lucentis. Not only that, those getting squalamine plus Lucentis were twice as likely to gain five or more lines of vision compared with the study’s monotherapy arm. Overall, patients treated with the combined topical/injectable regimen went home with a mean visual acuity gain of 10.4 letters compared with 6.3 for those treated only with Lucentis.1
The visual gains sometimes come swiftly. “One of the interesting things is that a lot of these patients recovered vision in the short term,” says Sherrol A. Reynolds, OD, associate professor at Nova Southeastern University College of Optometry. “The study was nine months, but some of them had improvement of their vision in four to eight weeks.”
While visual acuity is where the rubber hits the road, there was also improvement in physiological parameters. The combination treatment resulted in an average decrease of 139µm in central subfield thickness vs. 117µm in the monotherapy group. A series of cases reported last year were characterized by resolution of subretinal hyperreflective material as well as intraretinal and subretinal edema.2
This may look promising, but there’s a catch. Ohr’s primary endpoint in the study looked at the potential for reducing the number of injections needed to manage these patients optimally, and this didn’t happen. “The really disappointing fact is that it did not result in a decrease in the number of injections,” says Mark T. Dunbar, OD, director of optometry at the Bascom Palmer Eye Institute in Miami. “So, this is not a medication that can stand alone as monotherapy, or at least it would seem that way.”
Patients still receive the same amount of injections, albeit with some improvements in visual acuity because of the additional agent added to the regimen. “I’m not sure that it’s really significantly better,” Dr. Dunbar says, “but it is a little bit better in the eyes that received the combination.”
Phase III studies are set to begin sometime in the first half of this year. Here again the visual endpoints will be the same as those of Phase II, yet with a larger patient population.
|
|
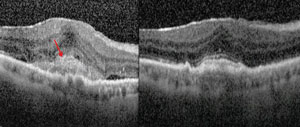
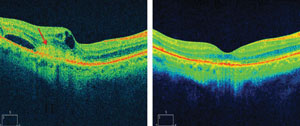
| Subretinal hyperreflective material is a marker for choroidal neovascular activity (images on right). Combination therapy with squalamine and ranibizumab resulted in the resolution of subretinal hyperreflective material as well as intraretinal and subretinal edema (images on left). The top images show a patient on combination therapy who had improvement of 21 letters of vision. A similar patient on the bottom improved by 26 letters. Photo: Ohr Pharmaceutical |
|
“The FDA has agreed to a nine-month primary efficacy endpoint based on the proportion of patients achieving a greater than or equal to three-line gain in visual acuity,” Dr. Chaglasian says. “Considering that the nine-month data of the Phase II study showed twice the number of patients who received squalamine twice daily with ranibizumab as needed achieved this goal compared to those receiving ranibizumab monotherapy, this would appear to be a very achievable endpoint.”
Dr. Chaglasian describes the upcoming study’s design and timeline. During the first year of the study, she says, patients will be randomized to receive monthly ranibizumab injections with squalamine drops twice daily or monthly ranibizumab injections with placebo eye drops. During the second year, they will receive ranibizumab injections as needed plus squalamine or placebo drops. “Though it will be a two-year study, the FDA will look at the data at nine months,” she says. “Considering this drug was fast-tracked in 2012, there is a possibility it will be approved prior to 2017.”
Purview of Optometry?
The most fundamental benefit of a potentially efficacious topical treatment for wet AMD, which is also readily amenable to patient satisfaction and compliance, is of course improved visual outcomes. Meanwhile, what might the prospective FDA approval of topical squalamine mean for the role of optometrists in managing these patients?
Dr. Chaglasian is optimistic. “The approval of a topical eye drop for AMD would be groundbreaking,” she says. “Up until now, once an optometrist detected wet AMD, that patient was most likely lost to our practice. We will no longer need to refer every patient for painful, costly, frequent injections to a retinal specialist. Having the capability to prescribe a safe, effective drop for this disease is equivalent to what we already do for our glaucoma patients. This would be an absolute win-win for our patients and our profession.”
Maybe so, but we still must await the Phase III results and further clinical experience with topical squalamine in treating wet AMD to gain a fuller sense of its ultimate implications for the OD’s role in managing these patients. Time will tell.
“I think as it stands now, this is going to remain a disease in the hands of the retinal specialists,” Dr. Dunbar says. Patients will still be followed closely by the treating ophthalmologist, as they’ll continue to need intravitreal injections on a regular basis. “If you’ve got a good relationship with a retinal specialist and you’re following a treat-and-extend protocol, an ophthalmologist or treating retinal specialist may be comfortable having an optometrist follow that patient until they develop fluid, and then at that point you refer them back,” he says. “But I don’t see a situation where an optometrist is going to be following these patients alone only on a topical eye drop, unless they get to a point where they’re stable.” He says the data have not yet borne out that possibility. Should that eventually happen, perhaps the patient may remain under the abiding purview of the optometrist’s care.
That is, the optometrist’s role in managing patients with wet AMD extends beyond treatment to the kinds of things they already do, namely diagnosis, monitoring and patient education.
For instance, we know that the AREDS study found that the long-term use of the antioxidant carotenoids lutein and zeaxanthin may forestall to some degree the progression of macular degeneration in some patients. Optometrists may wish to encourage patients to take these as supplements as well as to embrace a diet rich in antioxidants, including certain fruits and vegetables, especially dark, leafy ones.
Initial diagnosis and ongoing monitoring of wet AMD patients will remain key roles for optometrists. The use of spectral-domain ocular coherence tomography (SD-OCT) is a critical tool in doing so.
“You still need to closely monitor and educate patients, so they understand when there are changes in their vision,” Dr. Reynolds says. Instruct patients to regularly use the Amsler grid at home, for example.
‘It’s Getting Better All the Time’
Paul McCartney had reason for optimism when he wrote that lyric in 1967, and it’s not a stretch to say that optometrists have reason for, let’s say, measured optimism when contemplating potential advances in the management of wet AMD patients.
Topical squalamine may not represent the Holy Grail. Yet, its multi-pronged mechanism of anti-angiogenic activity coupled with its convenient and noninvasive delivery method suggest a potentially useful addition to the treatment of these patients, not to mention significant prospective cost savings in a global sense.
The potential impact on optometry may readily be inferred. “I would like to think that we could start prescribing the drop, and that would allow us to be more engaged with retinal specialists, and to follow these patients and comanage them with retinal specialists more,” Dr. Ferrucci says.
If topical squalamine is shown to improve visual outcomes in treating AMD in a safe, effective and patient-friendly manner, patients may stand to benefit in a significant way. Optometrists likewise figure to expand their role in managing these patients. And not just to expand this role, but to enjoy the satisfaction of seeing their patients experience better outcomes. Talk about a win-win scenario.
Robert Murphy is a freelance medical writer in Watertown, NY.
1. Abraham P. Interim Phase 2 Results of Squalamine Lactate Ophthalmic Solution 0.2% (OHR -102) In Neovascular Age-Related Macular Degeneration. Paper presented at American Academy of Ophthalmology 2014 Retina Subspecialty Day, October 18; Chicago.2. Ohrpharmaceutical.com. Press release: Ohr Pharmaceutical Announces Additional Squalamine Eye Drop (OHR-102) Phase II Clinical Data in Wet-AMD; The IMPACT Study. August 13, 2014.

