One of the most common questions we get from referring doctors regarding refractive surgeries is, “Which surgery is best for my patient?” Historically, this was not a difficult question, as all patients who had laser vision correction either underwent LASIK or PRK. Now, it’s become somewhat challenging with several procedures available today, from small-incision lenticule extraction (SMILE), implantable collamer lenses (ICL) and toric ICLs to the anticipated release of the Evo ICL, the trifocal IOLs, extended depth of focus IOLs and the light-adjustable lens (LAL) IOL.
The evaluation of a patient is vital and allows us to determine which procedure is most fitting, and then we set expectations. If we have a patient with great results but even higher expectations, we could end up with an unhappy patient. These procedures can have up to a 95% to 99% patient satisfaction rate.1-5
Let’s take a deeper dive into how to determine the best procedure for the patient, the current results we are getting for these different procedures and how to set expectations to have the happiest refractive surgery patients.
How Do We Choose?
In our clinic, we categorize every patient into one of the three milestones of vision development based on age:
• 18-40 years old: ocular adulthood/maturity. Most refractive errors have stabilized.
• 40-60 years old: dysfunctional lens syndrome/presbyopia. The midlife loss of near vision.
• 60 years old and up: cataracts.
Once a patient can identify which milestone they’ve reached, their visual goals are assessed, along with refractive error and ocular anatomy to determine their options. For example, a 24-year-old -6D patient whose myopia has fully stabilized is in their first ocular milestone. A patient like this has several options based on their anatomy and desires, including LASIK, SMILE, ICL and PRK.
So, how do we choose? The initial examination of these patients includes a dry and wet refraction, as well as a topography/tomography image with pachymetry and anterior chamber depth assessments.
When looking at the corneal tomography image, we assess the overall K values and look for any inferior steepening, thin pachymetry (<490µm) or abnormal posterior elevation (>15µm), any of which could be indicative of keratoconus or another corneal ectasia. Remember, in early keratoconus the posterior cornea protrudes forward, oftentimes preceding anterior corneal changes. Other factors to help rule out keratoconus patients for laser vision correction would be the Belin/Ambrosio enhance ectasia factor on the Pentacam (Oculus) or epithelial thickness mapping (Figure 1).
OCTs now have the ability to measure the thickness of the epithelium. Multiple studies have shown that the epithelium compensates for stromal changes, which means in keratoconus patients, thinning of the epithelium will occur. If we see any signs of inferior steepening, posterior float, abnormal Belin/Ambrosio enhance ectasia display thinning of the epithelium where the apex of the cone is, with thin pachymetry (<490µm), then we may wait on a vision correction surgery and instead consider crosslinking for this patient.
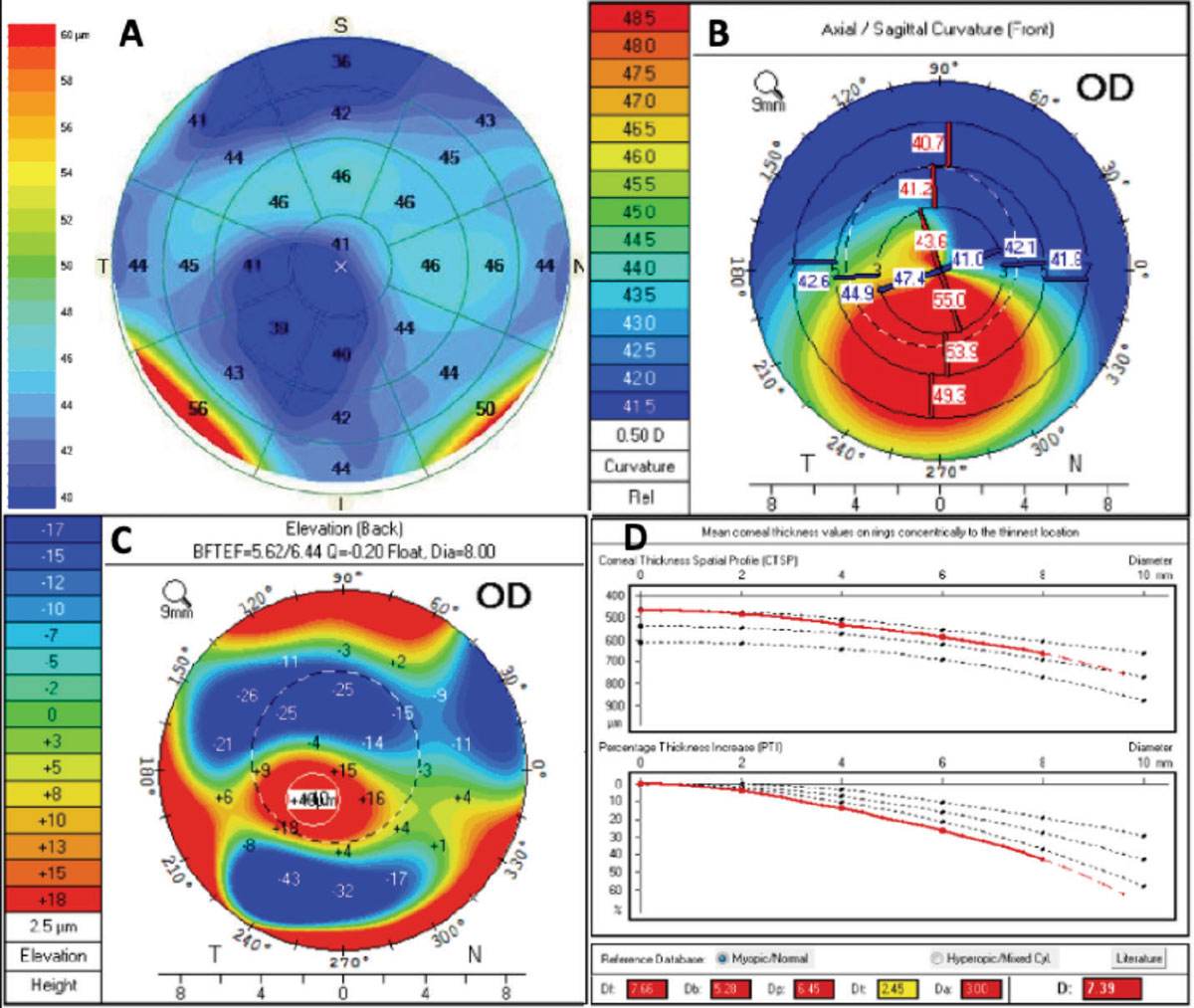 |
Fig. 1. This patient with keratoconus presented for a refractive surgery consult. OCT epithelial thickness (A) mapping showed thinning of the epithelium that corresponded with inferior steepening (B) and posterior float (C) on the pentacam image. The Belin/Ambrosio deviation display shows an abnormal “D” value consistent with keratoconus. Click image to enlarge. |
First milestone: ocular maturity
What surgery would we pick if this same 24-year-old -6D patient had a great corneal shape but had a corneal thickness of 475µm or less? In our practice, we would often rule out LASIK or SMILE because we prefer to see pre-op pachymetry greater than 480µm and a predicted residual stromal bed (RSB) thickness greater than 250µm. Some surgeons will use a RSB >300µm for LASIK patients to avoid the rare risk of inducing ectasia. If the patient has a cornea thinner than 480µm, we could consider PRK. With PRK, we prefer to maintain a RSB >400µm.
Another parameter we consider in our PRK algorithm is magnitude of myopia being treated. We are reluctant to treat myopia over -5D with PRK due to the increased risk of haze, even with using mitomycin-C during the surgery, not to mention the slower recovery time.6-8 Next, we look at the anterior chamber depth to determine if this patient could be a candidate for an ICL. The ICL can treat anywhere from 3D to 16D of myopia, and baseline corneal thickness is not a factor. ICLs are also approved for myopic reduction from -16 to -20D and can treat four diopters of cylinder. The ICL may be our leading option in this case. Other cases when to consider using an ICL would be abnormal topography, predicted RSB thickness <250µm, considerable preoperative dry eye disease and/or contact lens intolerance.
Second milestone: presbyopia
If a patient has passed their second milestone, we have two main sets of procedures to choose from: blended vision and refractive lens exchange. Blended vision is accomplished by correcting one of the eyes primarily for far, and the other eye is blended for middle and near depending on the residual accommodative amplitude we have to work with. Blended vision can be done with LASIK, SMILE, ICL and/or PRK.
Refractive lens exchange can be done with any of the current types of IOLs. The most popular lenses chosen for this are the trifocal or trifocal-like lenses (Synergy, Johnson & Johnson Vision; Panoptix, Alcon) that cover a complete range from near to far in both eyes. Trifocal lenses allow most patients to see better than 20/25 at distance, intermediate and near.4 Other patients consider using the extended depth of focus (EDOF) lenses (Eyhance, Johnson & Johnson Vision; Vivity, Alcon) which provide approximately 1.3 to 1.5D add.6
Patients sometimes choose to have one EDOF lens for distance (plano target) which achieves distance and intermediate range of vision, and the other blended (-0.50 to -1.25D target) to allow for the other eye set to see intermediate and near (Figure 2). The benefit of EDOF lenses is that the incidence of halos is less at the six-month mark compared to multifocal IOLs, especially those of the past. A study showed approximately 65% of patients reported little to no halos with the trifocal lens compared to approximately 85% of patients who reported little to no halos with EDOF lenses.9 If the patient is looking to be spectacle-independent as much as possible, the trifocal lenses are the way to go. Patients with trifocal lenses in both eyes are spectacle-independent 92% to 96% of the time, compared to 72% with EDOF lenses.10
With all of our refractive procedures, the assessment includes evaluating the overall media and a thorough retinal examination. The myopic patients that have retinal lesions could be at risk for rhegmatogenous retinal detachment (RRD). Refractive lens exchange (RLE) involves taking out the crystalline lens and putting in an IOL even in the absence of cataract. This could lead to volumetric changes in the eye—the crystalline lens is thicker than the IOL—leading to vitreous degeneration and movement that can predispose a patient to RRD. The risk of RRD after RLE is thought to be between 2% and 8% with a higher incidence found in patients who had pre-operative retinal lesions, capsular tear during surgery or lack of a PVD.11 For those who have retinal lesions, identifying these lesions preoperatively and recommending prophylactic treatment is best for the patient.12 Similar to with cataract surgery, these patients need regular follow-up postoperatively to evaluate their retina.
Third milestone: cataracts
Cataract surgery is still the most common refractive surgery we perform. With modern laser refractive cataract surgery, we have the ability to remove the cataract and improve vision by correcting astigmatism and presbyopia at the same time. If a patient wants to have cataract surgery to reduce their dependency on lenses, we use the same strategy with IOLs that we discussed with RLE procedures.
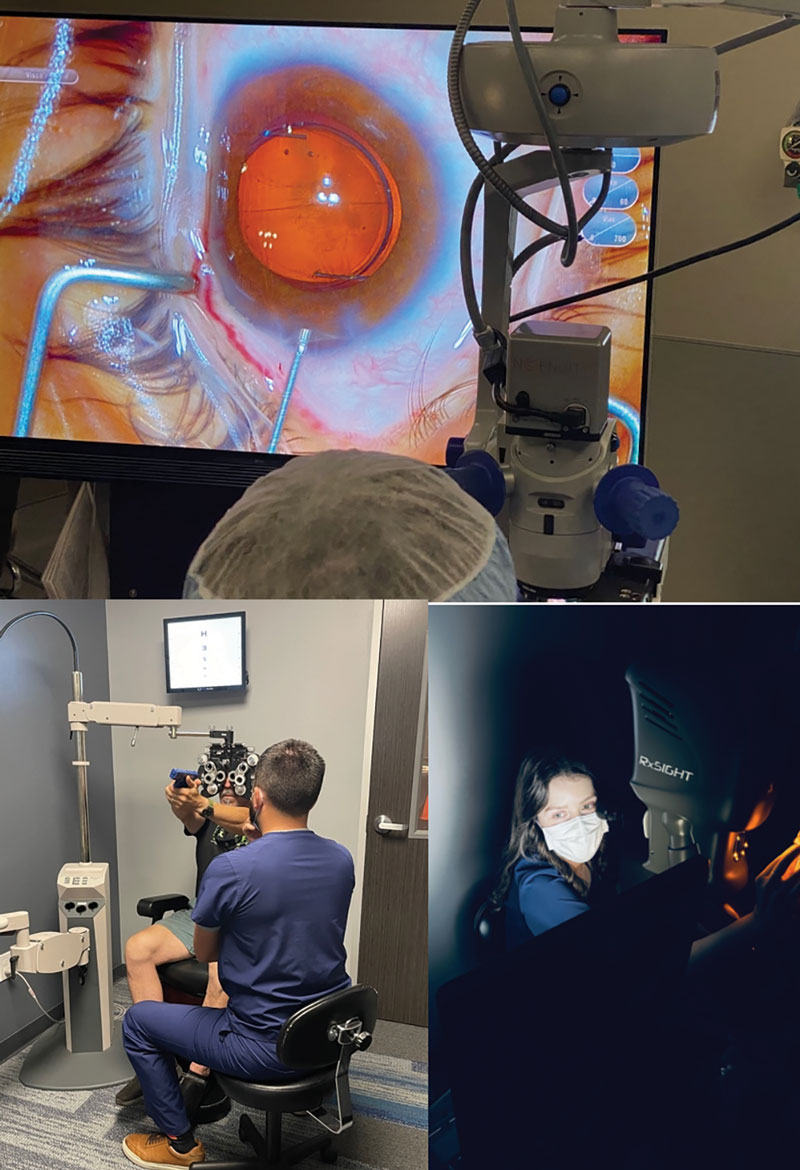 |
Fig. 2. Light-adjustable lens implantation (top) during cataract surgery allows for patients to adjust their refractive target after surgery. For example, a patient could want more near vision, and the light treatment may help them see their front sights for competitive shooting (bottom left), which is then locked in (bottom right). Click image to enlarge. |
Refractive Surgery Evaluation
It’s important to understand the patient’s visual demands, hobbies and complete eye anatomy when performing an evaluation. Is the myopic presbyopic patient who takes their glasses off to read really only wanting to correct their distance vision? Does the emerging emmetropic presbyope want perfect vision at all distances? Is the patient an up-and-coming MMA fighter who wants a LASIK alternative? Understanding the patient’s occupation and desires for what they want out of a treatment is very important. If they are wanting a procedure with a quick visual recovery, they should consider LASIK, SMILE or ICL instead of PRK, for example.
After getting a detailed history from the patient, the comprehensive anterior segment examination is next. The most common thing we see that changes the surgical plan are scars on the cornea from contact lenses. If a corneal opacity is present, a deeper LASIK interface could be employed. PRK could be performed and potentially remove some of the scar, or we could bypass touching the cornea altogether and consider an ICL.
ICLs are a great procedure for those with moderate to high amounts of myopia, especially when considering their future vision milestones. We know everyone will get cataracts if they live long enough. When a -9D patient has LASIK, the question is: will they be a candidate for a trifocal lens when they get cataracts several decades down the road? What would the spherical aberrations of the cornea measure after -9D LASIK? Some would think that due to the corneal appearance/aberrations, they would not be a candidate for a trifocal lens. In our practice, moderate to severe myopic patients typically choose ICLs because when they get cataract surgery as they get older, they can have the ICL and cataract removed, leaving an otherwise unaltered cornea. This maintains all options for any type of presbyopia-correcting IOL the patient may desire in the future.
“What would you do for a 9D myopic presbyope or emerging presbyope?” Remember that the ICL goes in front of the crystalline lens in the ciliary sulcus. Hence, we don’t see any posterior segment changes in volume, movement of the vitreous humor or resultant traction of the peripheral retina at the vitreous base. Refractive lens exchange in a -9D 41-year-old could potentially cause vitreoretinal changes; consequently, we typically choose the ICL for these patients, especially if they are younger than 50 with long axial lengths and increased risk for peripheral retinal pathology.
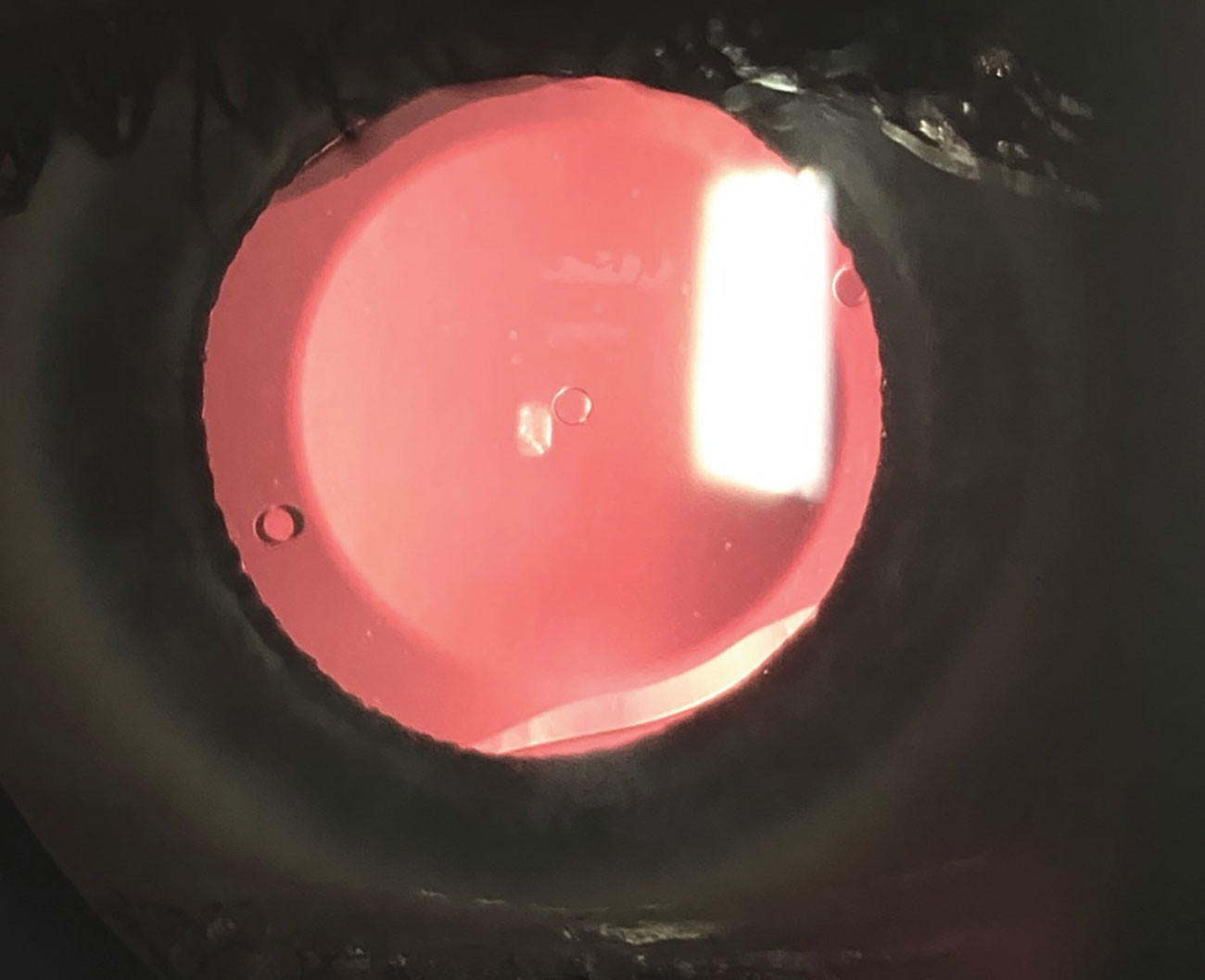 |
Fig. 3. A slit lamp photo shows the Evo ICL, which has a central port and will allow patients to have the ICL procedure without the need for an iridotomy. This will hopefully be approved soon by the FDA. Click image to enlarge. |
Setting Expectations
“Will I see 20/20 after the procedure? Will I go blind?” These are other common questions patients ask us. As far as visual acuity expectations, LASIK has come a long way the past 30 years and we have yet to find a case of someone going blind from LASIK. Way back in 1999, the data presented to the FDA to gain approval for LASIK showed a 50% chance of getting a patient to 20/20 vision. Fast-forward to the use of modern techniques and laser systems and nearly all patients can expect to achieve 20/20 uncorrected vision. In a recent study, 95% of patients were 20/20 after LASIK.13 SMILE is no different, with 95% of patients 20/20 one-day post-op.13 In our data presented at Optometry’s Meeting 2021, we observed that the patient’s vision was the same for the ICL procedure one day after surgery as well.14
Despite these incredibly safe and predictable outcomes with modern technology, managing post-op expectations is still very important. If a patient is not given proper expectations, they may think they are dealing with complications when in fact what they’re experiencing is a completely normal part of the recovery.
For our laser procedures, transient dryness is the most common symptom in the early preoperative period. Interestingly, the PROWL study found that patients are actually three times more likely to see improvement in dry eyes with modern LASIK compared to baseline in contact lenses.2 While this seems shocking at first, it is ultimately not surprising because we see patients coming in who seek an alternate solution for their vision due to dryness and intolerance to contact lens wear very commonly.
That said, dryness after LASIK—especially short-term—is the number one complaint we hear during the early post-op period. When treating the ocular surface appropriately both preoperatively and postoperatively, patients can obtain great outcomes as the tear film continues to improve during the first three to six months. Overall, we typically see less dryness with SMILE and ICL compared to LASIK, potentially due to less nerves being impacted during the procedure.13
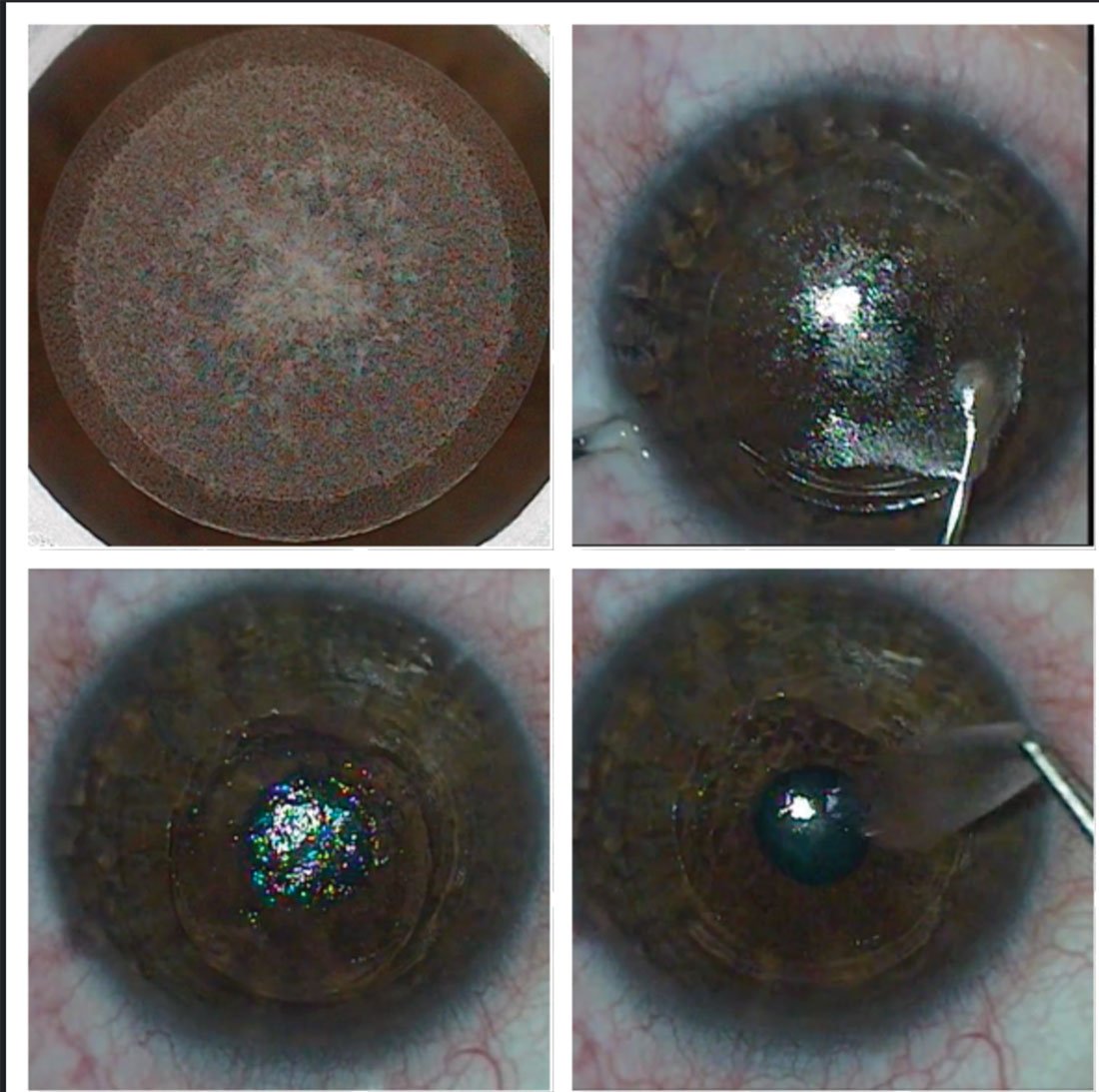 |
Fig. 4. The SMILE procedure creates (top left), dissects (top right) and removes a lenticule (bottom left and right) to correct myopia and myopic astigmatism. Click image to enlarge. |
Another benefit of SMILE is that the post-op restrictions are minimal due to use of a small incision, which limits loss of cornea integrity. Whereas we typically advise LASIK patients to stay out of public swimming water, avoid getting sweat in their eyes and avoid face makeup for at least a week, SMILE has almost no restrictions and patients are able to go back to all of those activities by the first day post-op.
Of all the different procedures in this group, the one that takes the longest to heal with the most hand-holding and reassurance is PRK. Patients need to know about recovery restrictions and plan to take a few days off from work during the first several days while the epithelium repopulates the surface of the cornea.
For our presbyopic patients, expectations around time needed for neuroadaptation is critical to success. For example, in our RLE and cataract patients, we inform them about the halos they can see at night from the diffractive rings of trifocal IOLs. The halos will continue to improve over time, generally within three to six months. Once they get used to headlight halos, these are some of the happiest patients we see. This clinical observation was evaluated in one study where functional MRI was used to assess neuroadaptation to multifocal IOLs.15 The study found that neuroadaptation occurred over time and was consistent with decreases in questionnaire symptom scores.15
Takeaways
Ultimately, we want happy patients. We know that as long as we take care of patients while managing their expectations, they will be ecstatic with the outcome. Marrying the right procedure and the right expectations with the right patient is the key to their happiness.
Dr. Saenz is the residency director and clinic director at Parkhurst NuVision and president of the Bexar County Optometric Society. He is a speaker for Biotissue, Zeiss, Johnson & Johnson, Lumenis and Glaukos, a researcher for Ocular Therapeutix, Novartis, Sun Pharma and Sight Sciences, and a consultant for LensAR and Quidel.
Dr. Vanrachack and Dr. Wiechmann are assistant adjunct clinical professors at the University of the Incarnate Word Rosenberg School of Optometry and faculty of the Refractive Surgery Alliance Grands Rounds Collaborative Care Series.
All three practice at Parkhurst NuVision, an OD-MD integrated care practice that specializes in refractive surgery, and are Fellows of the American Academy of Optometry.
| 1. Price MO, Price DA, Bucci FA, Jr., et al. Three-year longitudinal survey comparing visual satisfaction with LASIK and contact lenses. Ophthalmology. 2016;123(8):1659-66. 2. Eydelman M, Hilmantel G, Tarver ME, et al. Symptoms and satisfaction of patients in the patient-reported outcomes with laser in situ keratomileusis (PROWL) studies. JAMA Ophthalmol. 2017;135(1):13-22. 3. Solomon KD, Fernandez de Castro LE, et al. LASIK world literature review: quality of life and patient satisfaction. Ophthalmology. 2009;116(4):691-701. 4. Ferreira TB, Ribeiro FJ, Silva D, et al. Compaarsion of refractive and visual outcomes of three presbyopia-correcting intraocular lenses. J Cataract Refract Surg. July 21, 2021. [Epub ahead of print.] 5. Sarver EJ, Sanders DR, Vukich JA. Image quality in myopic eyes corrected with laser in situ keratomileusis and phakic intraocular lens. J Refract Surg. 2003;19(4):397-404. 6. Gambato C, Ghirlando A, Moretto E. Mitomycin C modulation of corneal wound healing after photorefractive keratectomy in highly myopic eyes. Ophthalmology. 2005;112(2):208-18. 7. Carones F, Vigo L, Scandola E, Vacchini L. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy. J Cataract Refract Surg. 2002;28(12):2088-95. 8. Shalaby A, Kaye GB, Gimbel HV. Mitomycin C in photorefractive keratectomy. J Refract Surg. 2009;25(1 Suppl):S93-7. 9. Rampat R, Gatinel D, Multifocal and extended depth-of-focus intraocular lenses in 2020, Ophthalmology. 2021;128(11):e164-85. 10. Rodov L, Reitblat O, Levy A. Visual outcomes and patient satisfaction for trifocal, extended depth of focus and monofocal intraocular lenses. J Refract Surg. 2019;35(7):434-40. 11. Rosen E. Risk management for rhegmatogenous retinal detachment following refractive lens exchange and phakic IOL implantation in myopic eyes, J Cataract Refract Surg. 2006;32(5):697-701. 12. Arevalo JF, Lasave AF, Torres F, Suarez E. Rhegmatogenous retinal detachment after LASIK for myopia of up to –10 diopters: 10 years of follow-up. Graefes Arch Clin Exp Ophthalmol. 2012. 250(7):963-70. 13. Hamilton DR, Chen AC, Khorrami R, et al. Comparison of early visual outcomes after low-energy SMILE, high-energy SMILE, and LASIK for myopia and myopic astigmatism in the United States. J Cataract Refract Surg. 2021;47(1):18-26. 14. Saenz B, Mueller B, Abraham N, Parkhurst G. Toric ICL: early United States experience 2018. Poster. Optometry’s Meeting 2021. 15. Rosa AM, Miranda ÂC, Patrício MM, et al. Functional magnetic resonance imaging to assess neuroadaptation to multifocal intraocular lenses. J Cataract Refract Surg. 2017;43(10):1287-96. |

