Historically, intraocular pressure (IOP) and its role in glaucoma boiled down to a simple equation: high pressure equals glaucoma diagnosis. Although IOP remains the most important and only modifiable risk factor in glaucoma care, modern research indicates that the historical formula is more complex than initially understood.
At present, a number of recognizable forces influence the retinal ganglion cells and optic nerve head (ONH). These include IOP, cerebrospinal fluid pressure (CSFP) and ocular perfusion pressure (OPP). The interrelations between these forces are complicated, and our understanding of each of their roles is constantly evolving.
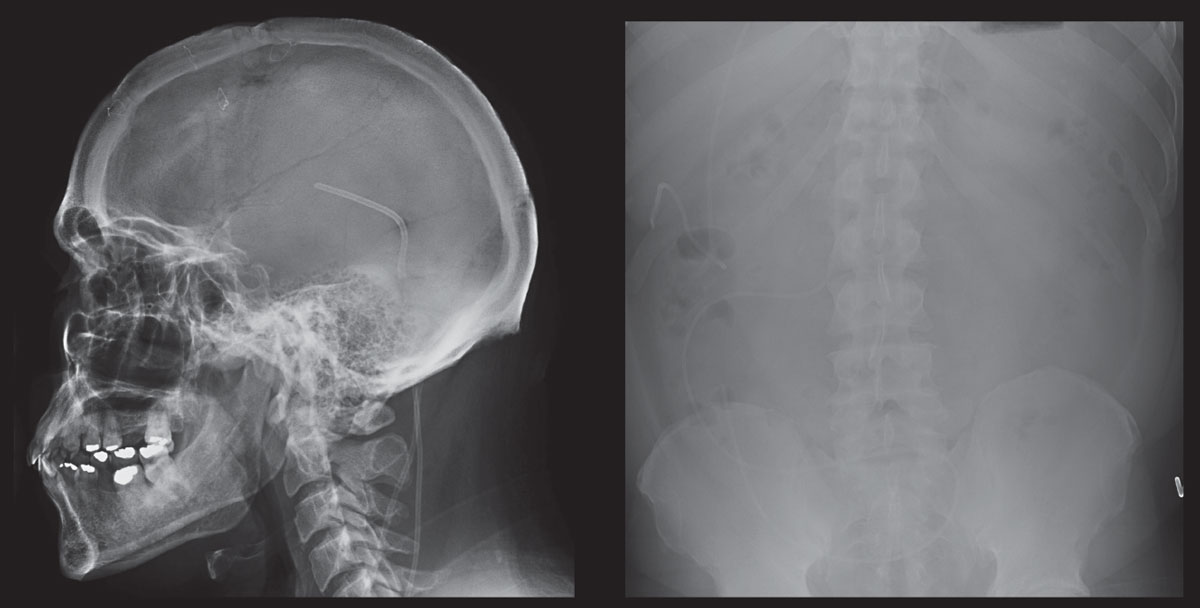 |
| These scans show skull and abdomen radiographs of a patient who is post-ventriculoperitoneal shunt placement for pathological increase in CSF. Click image to enlarge. |
Pressure Differences
IOP—taken with conventional Goldmann applanation tonometry (GAT)—is often interpreted as an accurate representation of the pressure within the eye. However, what is measured by conventional force tonometry is the transcorneal pressure difference (where force applied outside the eye is equal to force inside the eye, as measured across the cornea) and not truly representative of IOP.1,2 In fact the absolute pressure in the eye involves atmospheric pressure and is calculated as the sum of transcorneal pressure and atmospheric pressure (standard atmospheric pressure at sea level is 760mm Hg).
For example, if an eye is measured at 15mm Hg using GAT, the actual pressure is 775mm Hg (760mm Hg +15mm Hg). For an eye measuring 30mm Hg by GAT, it is 790mm Hg (760mm Hg + 30mm Hg). Some may infer that if an eye’s GAT pressure measures 30mm Hg it is twice as great as one measured at 15mm Hg. However, in this example, on an absolute level the pressure difference is only 2.5%.2
To further advance this notion of absolute pressure and its effect on the eye, consider that no evidence shows that scuba divers exposed to increased atmospheric pressures experience acutely increased eye pressure leading to glaucoma, nor that mountain climbers at decreased atmospheric pressure are protected from the disease. The transcorneal pressure does not change under either of the above circumstances.2 This knowledge led to the proposal that glaucoma is not influenced only by pressure in the eye, but rather by other relative systemic pressure imbalances.3
What this all means is that transcorneal pressure difference (TCPD) may not be the most critical metric in glaucoma. ONH damage may be most dependent upon the translaminar pressure difference (TLPD). However, the TCPD may be able to function as a surrogate for TLPD, which is currently difficult to directly assess.1 The lamina cribrosa (LC) also contains fenestrations that transmit optic nerve axons and is thought to be the primary site of glaucomatous damage.4 Thus, the pressure gradient across the LC is of significant interest.
To understand the TLPD and its proposed role in ONH pathology, first understand that the LC functions as an anatomical barrier between the intraocular and retrolaminar (subarachnoid) spaces.2,5 IOP in the intraocular space and intracranial pressure (ICP) in the retrolaminar space are interrelated and exist as relatively independent pressurized systems, maintaining equilibrium through their respective aqueous and cerebral spinal fluid (CSF) circulations.6 In its function as a barrier, it has been proposed that the LC may prevent leakage of aqueous humor from the intravitreal space into the retrobulbar CSF surrounding the retrobulbar portion of the optic nerve, and vice versa.2
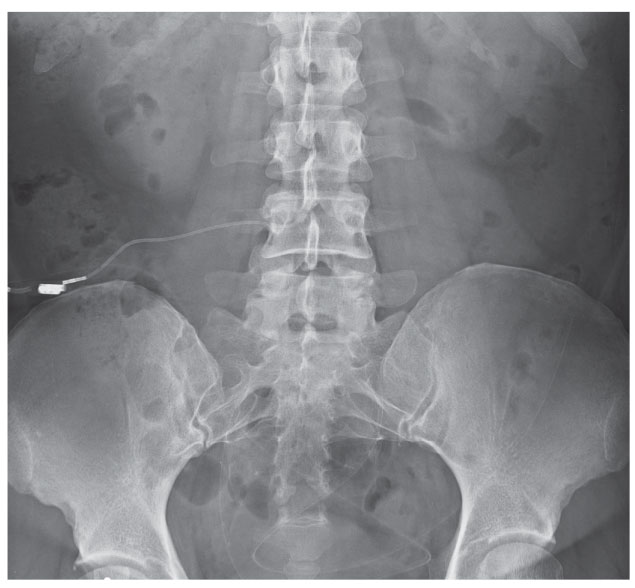 |
| This anteroposterior radiograph shows the lumbosacral region of a 43-year-old patient with a history of intractable IIH having undergone lumboperitoneal shunt placement. Had this case occurred presently, this patient may have undergone transverse sinus stenting. |
Translaminar Pressure
The average TCPD by GAT is 10mm Hg to 21mm Hg, and normal CSFP is 5mm Hg to 15mm Hg. In general, a mean positive pressure difference of 4mm Hg is directed posteriorly across the LC.3,7 This is known as the translaminar pressure gradient (TPG). The TPG depends on the TLPD and the distance between the intraocular and retrobulbar space, with this distance between the compartments highly dependent on axial thickness of the LC.7 Under normal conditions, there is continuous ortho and retrograde axoplasmic transport of small and large molecules within the ONH. Any imbalance in this normal pressure gradient may result in constriction of axoplasmic flow, LC deformation, altered blood flow and subsequent damage to ONH axons.6,7
The role of TPG and ICP in the development of glaucoma is the focus of recent research.7 A 2015 meta-analysis shows that patients with normal tension glaucoma (NTG) and high tension glaucoma (HTG) have an abnormally high TPD compared with healthy subjects, presumably due to lower CSFP specifically in the NTG group.6 Another study reviewing medical records from patients undergoing lumbar puncture shows a substantial reduction in CSFP beginning in the sixth decade of life. Given that the prevalence of glaucoma increases with age while the CSFP decreases, researchers hypothesize that lower CSFP has a deleterious effect on the ONH.8 Conversely, an abnormally high CSFP exists in patients with ocular hypertension (OHT), resulting in a normal TPG in spite of higher IOP.9,10 Accordingly, the pressure differential at the LC may be protective in OHT, but detrimental in NTG and HTG.
The current challenge with measuring a patient’s ICP is the invasiveness of conventional measuring techniques.6 Newer studies suggest alternative noninvasive methods for measuring ICP, such as two-depth transcranial doppler ultrasound.6 Although exciting, such techniques are not yet commercially available. In addition to CSFP being difficult to directly measure and inaccurate to estimate, we currently do not have good therapy to increase CSFP as a treatment for glaucoma.
Fortunately, research continues to improve our understanding of CSF dynamics, and treatment may be on the horizon. Ongoing safety trials are evaluating pressurized goggles (Multipressure Dial, Equinox) whose goal is to restore balance in the pressure differential between IOP and CSFP in patients with ONH diseases pathologically affected by known or presumed imbalance.11 These goggles are designed to create a negative pressure over the eye to decrease IOP and restore normal IOP/CSFP balance.
Elevated CSFP
In situations when the CSFP is abnormally high, the TPG is pathologically altered, with forces into the eye exceeding those going out, producing anterograde axoplasmic stasis and subsequent ONH edema.12 This is seen in cases of idiopathic intracranial hypertension (IIH), where bilateral ONH edema may result in axonal loss, optic atrophy and subsequent permanent vision loss with reduced quality of life.13
IIH is most prevalent in women of childbearing age who are obese. Obesity increases the risk of developing IIH 20-fold.14 The pathogenesis of IIH is unknown, with multiple proposed theories, including CSF hypersecretion, obstruction of outflow and decreased CSF reabsorption and increased venous pressure.
Venous studies show IIH patients often have stenosis along the transverse-sigmoid sinus junction, either bilaterally or involving the dominant sinus. It is unknown whether this stenosis is intrinsically due to sinus wall abnormalities or extrinsically a result of compression due to increased CSF. Regardless, both can result in reduced CSF absorption and subsequent significant patient symptoms.13 Although this association between IIH and venous sinus stenosis does not prove a causal link, it has provided an additional treatment method for patients with intractable disease.
Optimal management is based on treating the underlying disease, protecting vision and minimizing disabling headaches.15 Unless vision loss is imminent, medical management and weight loss prevail as initial therapies.
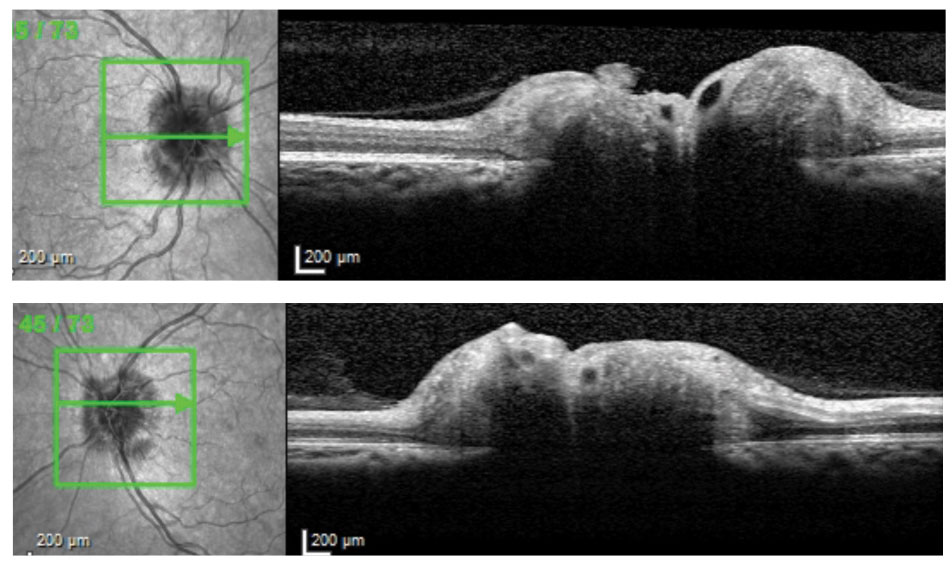 |
| Cross-sectional OCT slices through ONHs of 38-year-old patient with IIH and opening CSF pressure 38cm H2O. |
Corneal Hysteresis
A major confounder in the use of TCPD as a surrogate for TLPD is that conventional Goldmann tonometry is limited by capturing only force applied and not corneal dynamics.16 Goldmann was aware that corneal thickness and elastic properties might influence readings, but the ability to record these measurements only became available recently. It is accepted that thin corneas can lead to underestimation of IOP and thick corneas to overestimation.17
Although central corneal thickness (CCT) is a corneal dimension, it is not a biomechanical property that limits its utility.18 The viscoelastic cornea can be more accurately characterized by hysteresis.19 This is a measurement capturing how a material or system adapts to the loading and unloading of a force applied to it.19 Corneal hysteresis (CH) reflects the cornea’s ability to absorb and release energy created by applanation forces during measurement.19,20
Similarly, glaucoma may involve the biomechanical properties of the ONH, specifically the LC, scleral canal wall and peripapillary sclera.21 Researchers propose that corneal biomechanics might reflect the constitution of the extracellular matrix (ECM). Given that the cornea, sclera, peripapillary ring and LC are essentially derived from the same ECM, it is reasonable to postulate that their biomechanical properties are similar.22 Accordingly, CH could reflect the ONH’s ability to resist deformation from the various confounding pressures. A high CH would thus confer a protective effect whereas a low CH would increase susceptibility to damage.23
Research suggests a lower CH in glaucoma patients vs. glaucoma suspects and normals.24 Low CH is also associated with progression of glaucoma and an increased rate of progression.25-29 Furthermore, that association is more powerful than the association with CCT.25-29 Used in isolation, one report found a CH average of 7.5mm Hg±1.4mm Hg in progressors versus 9.0mm Hg±1.8mm Hg with stable disease.28 Research shows that for every 1mm Hg decrease in CH from baseline there was an associated 0.25% per year increased rate of visual field index decline.
Like other measurements, CH suffers from measurement noise and is directly influenced by CCT.23 It is also inversely influenced by IOP.23 Thus, both influences should be considered when interpreting CH. One study evaluated the relationship in a study of patients with primary open-angle glaucoma (POAG) and OHT. They found that, as CCT increases, so does CH. Specifically, patients with POAG and thinner corneas maintained lower CH levels than those with intermediate and thicker corneas.
The authors interpreted these findings as suggestive that CH in isolation, without consideration of CCT, could erroneously suggest or mask glaucoma risk. Combining the two improves the sensitivity of diagnosis beyond either individually.23
As with IOP, CH can change over time. Studies suggest an inverse relationship: CH is lower as IOP increases, and can become higher as IOP lowers.19 All this information underscores the realization that reliance on the TCPD alone is insufficient in risk assessment.
At present, the Ocular Response Analyzer (Reichert Technologies) is the only commercially available product that can capture corneal hysteresis in office.30,31
 |
| Click image to enlarge. |
What Causes IOP to Rise?
In a normal eye, aqueous humor (AH) flow against resistance generates the IOP. IOP increases with either overproduction of AH or a compromised outflow. Two pathways exist for AH drainage: Conventional/pressure-sensitive (major) and unconventional/pressure-insensitive (minor). The conventional pathway consists of the trabecular meshwork, Schlemm’s canal and collector channels. Interestingly, the discovery of AH atherosclerotic plaque markers in glaucoma patients supports the view that the anterior chamber is a specialized vessel where corneal and trabecular endothelial cells form the walls and whose “blood” is the AH.32
The second, unconventional, pathway—frequently referred to as uveoscleral outflow—represents nontrabecular clearance of AH and is composed of the uveal meshwork and anterior face of the ciliary muscle.33 Thus, this pathway lacks recognizable channels and vessels.
Pressure Fluctuations
IOP is a dynamic parameter that varies spontaneously over time. As such, it is subject to short- and long-term diurnal and nyctohemeral fluctuation.43 IOP regulation is complicated. It can be influenced by activity, body positioning (supine vs. standing, or sitting), nocturnal elevation, hypo- or hyper-response to medications and other factors.35,36 IOP fluctuation, peak IOP or the combination of the two play a prominent role in the development and progression of glaucomatous damage, with more recent studies suggesting peak IOP to be the best predictive factor.37-42
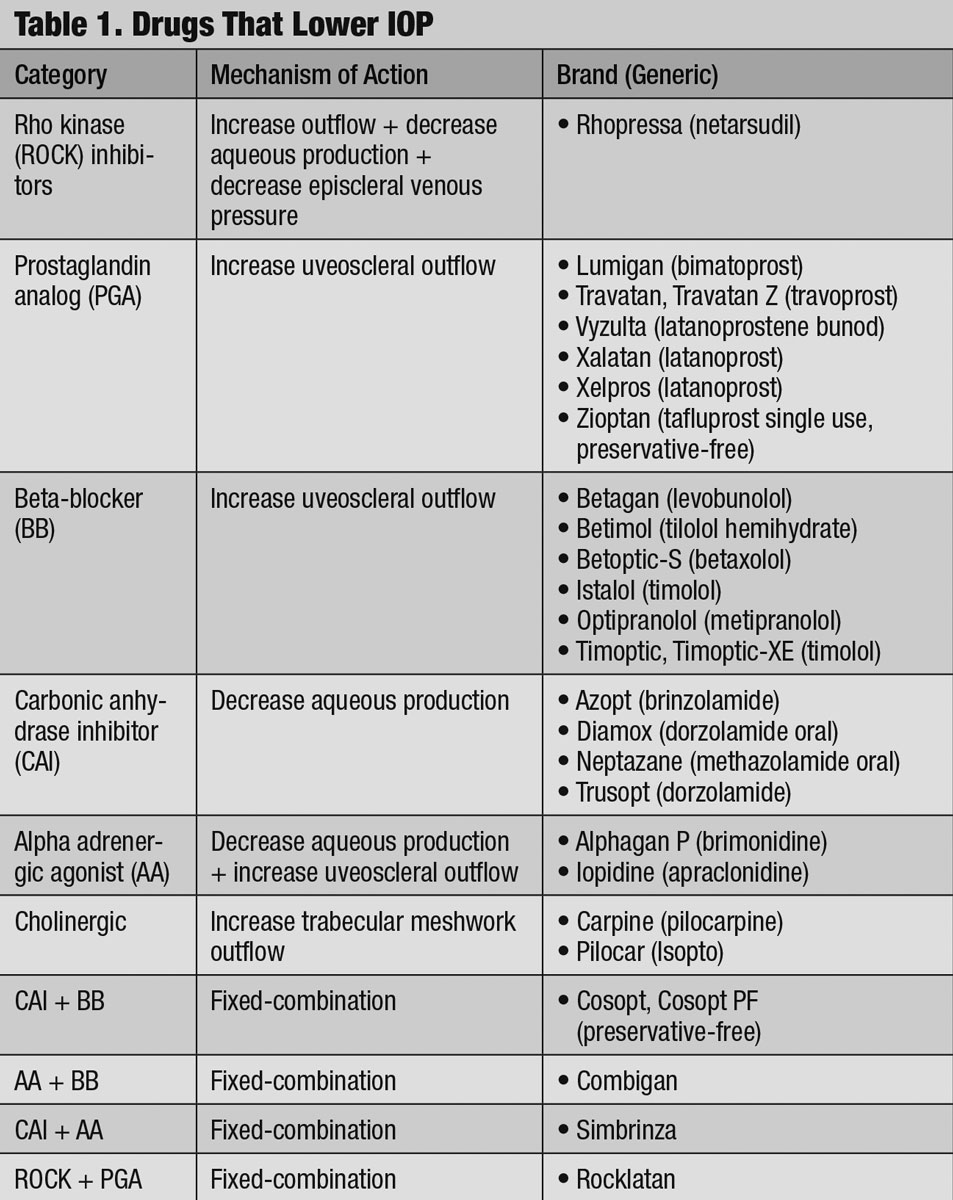
Understanding and accounting for these dynamics becomes important when regulating IOP with commerically available drugs (Table 1).
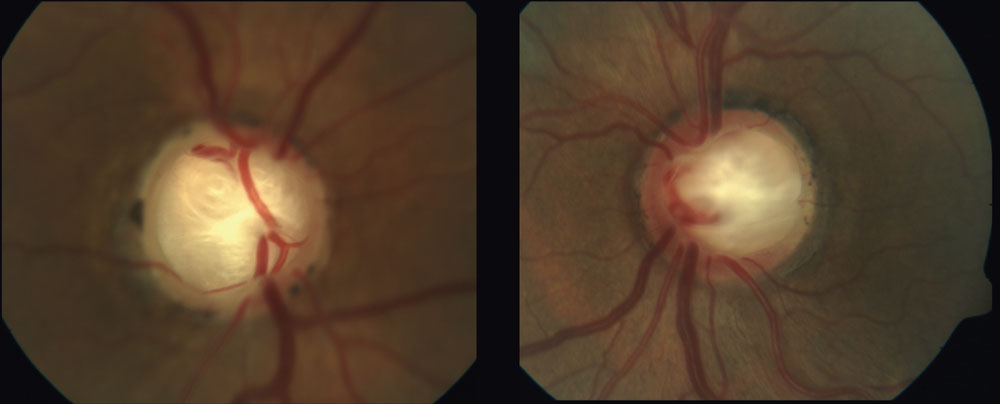 |
| Deep laminar cupping can be seen in this 70-year-old patient whose in-office IOPs never exceeded 19mm Hg. Lower CSFP with subsequent alteration to the TLPD may have resulted in the substantial excavation in this NTG case. Click image to enlarge. |
Ocular Perfusion Pressure
Several studies have suggested associations between vascular factors and glaucoma.43 OPP refers to the pressure available to move blood through ocular vasculature, with lower OPP presumably resulting in less blood flow to the ONH and subsequent ischemic damage.
Perfusion pressure of an organ is the pressure difference between its arterial and venous supply. This does not necessarily hold true for the eye, as there are other factors at play. Historically, the simple calculation of blood pressure (BP) minus IOP has been employed to reflect OPP.43 Recent research suggests this calculation should be abandoned, as it may oversimplify OPP’s complexity.44
Studies show a correlation between glaucoma and both low and fluctuating OPP, where the greater the fluctuation or the lower the OPP the greater risk of damage.45-47 One study specifically shows a diastolic perfusion pressure (diastolic BP minus IOP) of 56mm Hg or lower to be a useful threshold to help clinically identify patients at risk for progressive glaucomatous optic neuropathy.45-47
Conversely, a 2015 study found that although there appears to be a relationship between TLPD and optic nerve damage, there was no support for the notion that OPP plays a major role in the pathogenesis of optic neuropathy. The authors calculated OPP as follows:45
OPP = 2/3 [diastolic BP + 1/3 (systolic BP - diastolic BP)] - IOP
Undoubtedly, dynamics within and between the different interrelated measurements (CSF pressure, IOP and TLPD) provide uncertainty when attempting to calculate and implement OPP. Pulse-related issues can increase the CSF in relation to IOP, so that TLPD decreases. If, for a brief moment, the orbital CSF pressure becomes higher than IOP, than TLPD becomes reversed. This can result in a change in the undulation of the TLPD, with consequences for axoplasmic flow entering the eye.45
In addition to pulse-related changes, both IOP and CSF pressure change in relationship to body position.45 Circadian variations in OPP change as BP dips during sleep while IOP is at its peak. This places the ONH in a potentially more vulnerable state. Ambulatory BP monitoring can help identify patients who are “dippers” and at a higher theoretical risk from advancing glaucomatous damage.48 A recent publication demonstrated that the extent of the dip in diastolic BP is a significant predictor of subsequent visual field progression in patients with NTG.49
Another consideration is the effect of systemic or topical medication when attempting to determine OPP. It decreases when systemic BP is lower or when IOP is higher. Attempts to modify either of these conditions with medication affect the overall equation. Ultimately, a team approach with the patient’s primary care physician is advisable when managing patients with BP issues and glaucoma.48
Undoubtedly, the eye is exposed to many forces, creating a complex relationship that, when imbalanced, can result in glaucomatous optic neuropathy. Increased knowledge and awareness of these various pressures will only increase our capacity to provide more customized management of our patients.
Dr. Sanderson is associate professor and assistant director of residency programs at Southern College of Optometry.
Dr. Rixon is an attending at the Memphis VA and is a member of the Optometric Glaucoma Society.
Dr. Williamson is the residency supervisor at the Memphis VA Medical Center and is a member of the Optometric Retina Society.
| 1. Berdahl J. Cerebrospinal fluid pressure and glaucoma. Glaucoma Today. glaucomatoday.com/2009/10/GT1009_02.php/. October 2009. Accessed April 22, 2019. 2. Jonas J. Role of cerebrospinal fluid pressure in the pathogenesis of glaucoma. Acta Ophthalmologica. 2011;89(6):505-14. 3. Berdahl J, Allingham R. Intracranial pressure and glaucoma: Curr Opin in Ophthalmol. 2010;21(2):106-11. 4. Wostyn P, Van Dam D, De Deyn P. Intracranial pressure and glaucoma: Is there a new therapeutic perspective on the horizon? Medical Hypotheses. 2018;118:98-102. 5. Fleischman D, Berdahl J, Stinnett S, et al. Cerebrospinal fluid pressure trends in diseases associated with primary open‐angle glaucoma. Acta Ophthalmologica. 2015;93(3):e234-6. 6. Siaudvytyte L, Januleviciene I, Ragauskas A, et al. Update in intracranial pressure evaluation methods and translaminar pressure gradient role in glaucoma. Acta Ophthalmologica. 2015;93(1):9-15. 7. Guy A, Wiggs J, Turalba A, Pasquale L. Translating the low translaminar cribrosa pressure gradient hypothesis into the clinical care of glaucoma. Seminars in Ophthalmol. 2016;31(1-2):131-9. 8. Fleischman D, Berdahl J, Zaydlarova J, et al. Cerebrospinal fluid pressure decreases with older age. Zheng Y, ed. PLoS ONE. 2012;7(12):e52664. 9. Ren R, Zhang X, Wang N, et al. Cerebrospinal fluid pressure in ocular hypertension. Acta Ophthalmologica. 2011;89(2):e142-e148. 10. Berdahl J, Fautsch M, Stinnett S, Allingham R. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma and ocular hypertension: a case-control study. Invest Ophthalmol Vis Sci. 2008;49(12):5412-8. 11. Equinox Balance Goggles: The effects of local orbital pressure changes on intraocular pressure. NSBRI. nsbri.org/researches/equinox-balance-goggles-the-effects-of-local-orbital-pressure-changes-on-intraocular-pressure/. Accessed April 22, 2019. 12. Radke P, Rubinstein T, Hamilton S, et al. The translaminar pressure gradient: papilledema after trabeculectomy treated with optic nerve sheath fenestration. J Glaucoma. 2018;27(10):e154-7. 13. Madriz Peralta G, Cestari D. An update of idiopathic intracranial hypertension: Curr Opin Ophthalmol. 2018;29(6):495-502. 14. Giridharan N, Patel SK, Ojugbeli A, et al. Understanding the complex pathophysiology of idiopathic intracranial hypertension and the evolving role of venous sinus stenting: a comprehensive review of the literature. Neurosurgical Focus. 2018;45(1):E10. 15. Mollan S, Davies B, Silver N, et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neuro, Neurosurg Psychiatry. 2018;89(10):1088-100. 16. Whitacre M, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol. 1993;38(1):1-30. 17. Stamper RL. A history of intraocular pressure and its measurement. Optom Vis Sci. 2011;88(1):E16-28. 18. Sng C, Ang M, Barton K. Central corneal thickness in glaucoma. Curr Opin Ophthalmol. 2017;28(2):120-126. 19. Deol M, Taylor D, Radcliffe N. Corneal hysteresis and its relevance to glaucoma. Curr Opin Ophthalmol. 2015;26(2):96-102. 20. Murphy M, Pokrovskaya O, Galligan M, O’Brien C. Corneal hysteresis in patients with glaucoma-like optic discs, ocular hypertension and glaucoma. BMC Ophthalmol. 2017;17(1):1. 21. Park J, Jun R, Choi K. Significance of corneal biomechanical properties in patients with progressive normal-tension glaucoma. Bri J Ophthalmol. 2015;99(6):746-51. 22. Wells A, Garway-Heath D, Poostchi A, et al. Corneal hysteresis but not corneal thickness correlates with optic nerve surface compliance in glaucoma patients. Invest Ophthalmol Vis Sci. 2008;49(8):3262-8. 23. Pensyl D, Sullivan-Mee M, Torres-Monte M. Combining corneal hysteresis with central corneal thickness and intraocular pressure for glaucoma risk assessment. Eye (Lond). 2012;26(10):1349-56. 24. Sullivan-Mee M, Billingsley SC, Patel AD, et al. Ocular Response Analyzer in subjects with and without glaucoma. Optom Vis Sci. 2008;85(6):463-70. 25. Congdon N, Broman AT, Bandeen-Roche K. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol. 2006;141(5):868-75. 26. Medeiros F, Meira-Freitas D, Lisboa R, et al. Corneal hysteresis as a risk factor for glaucoma progression: A prospective longitudinal study. Ophthalmol. 2013;120(8):1533-40. 27. Park J, Jun R, Choi K. Significance of corneal biomechanical properties in patients with progressive normal-tension glaucoma. Br J Ophthalmol. 2015;99(6):746-51. 28. De Moraes C, Hill V, Tello C, et al. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J Glaucoma. 2012;21(4):209-13. 29. Chee R, Silva F, Ehrlich J, Radcliffe N. Agreement of flicker chronoscopy for structural glaucomatous progression detection and factors associated with progression. Am J Ophthalmol. 2013;155(6):983-90. 30. Nakao Y, Kiuchi Y, Okimoto S. A comparison of the corrected intraocular pressure obtained by the Corvis ST and Reichert 7CR tonometers in glaucoma patients. PLoS ONE. 2017;12(1):e0170206. 31. Susanna B, Ogata N, Daga F. Association between rates of visual field progression and intraocular pressure measurements obtained by different tonometers. Ophthalmol. 2019;126(1):49-54. 32. Saccà S, Pulliero A, Izzotti A. The dysfunction of the trabecular meshwork during glaucoma course. J Cell Physiol. 2015;230(3):510-25. 33. Goel M, Picciani R, Lee R, Bhattacharya S. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010;4:52-59. 34. Realini T, Weinreb R, Weinreb N, Wisniewski S. Short-term repeatability of diurnal intraocular pressure patterns in glaucomatous individuals. Ophthalmol. 2011;118(1):47-51. 35. Malihi M, Sit A. Effect of head and body position on intraocular pressure. Ophthalmol. 2012;119(5):987-91. 36. Ittoop S, SooHoo J, Seibold L. Systematic review of current devices for 24-h intraocular pressure monitoring. Adv Ther. 2016;33(10):1679-90. 37. Asrani S, Zeimer R, Wilensky J, et al. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9(2):134-42. 38. Konstas A, Quaranta L, Mikropoulos DG, et al. Peak intraocular pressure and glaucomatous progression in primary open-angle glaucoma. J Ocul Pharmacol Ther. 2012;28(1):26-32. 39. De Moraes C, Juthani V, Liebmann J, et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol. 2011;129(5):562-8. 40. Jonas J, Budde W, Stroux A, et al. Diurnal intraocular pressure profiles in chronic open-angle glaucoma. Asia Pac J Ophthalmol. 2012;1(2):84-7. 41. Gardiner S, Johnson C, Demirel S. Factors predicting the rate of functional progression in early and suspected glaucoma. Invest Ophthalmol Vis Sci. 2012;53(7):3598-604. 42. Susanna R, Clement C, Goldberg I, Hatanaka M. Applications of the water drinking test in glaucoma management. Clin Experiment Ophthalmol. 2017;45(6):625-31. 43. Costa V, Harris A, Anderson D, et al. Ocular perfusion pressure in glaucoma. Acta Ophthalmol. 2014;92(4):e252-66. 44. Khawaja A, Crabb D, Jansonius N. Time to abandon over-simplified surrogates of ocular perfusion pressure in glaucoma research. Acta Ophthalmol. 2015;93(1):e85-6. 45. Jonas J, Wang N, Nangia V. Ocular perfusion pressure vs. estimated trans-lamina cribrosa pressure difference in glaucoma: the central India eye and medical study (an American ophthalmological society thesis). Trans Am Ophthalmol Soc. 2015;113:T6. 46. de Oliveira APC, Kasahara N. Correlation between ocular perfusion pressure fluctuation and glaucoma severity. Int Ophthalmol. 2015;35(2):187-92. 47. Quaid P, Simpson T, Freddo T. Relationship between diastolic perfusion pressure and progressive optic neuropathy as determined by Heidelberg retinal tomography topographic change analysis. Invest Ophthalmol Vis Sci. 2013;54(1):789-98. 48. Levine R, Yang A, Brahma V, Martone J. Management of Blood Pressure in Patients with Glaucoma. Curr Cardiol Rep. 2017;19(11):109. 49. Kwon J, Jo Y hye, Jeong D, et al. Baseline systolic versus diastolic blood pressure dip and subsequent visual field progression in normal-tension glaucoma. Ophthalmol. 2019;8. |

