Mind-Eye ConnectionThe October 2024 issue of Review of Optometry focuses on the treatment and management of neuro-ophthalmic disorders. Check out the other articles featured in this issue: |
Three of the 12 cranial nerves (CNs) are dedicated to the innervation of six paired extraocular muscles. Disruption in the nerve signal results in ocular motility dysfunction and diplopia. Below, we provide an overview of isolated oculomotor, trochlear and abducens nerve palsies and discuss the common etiologies, unique clinical findings and management strategies of each. The goal is to equip you with the knowledge you need to feel confident monitoring these patients and providing them with solutions to improve their visual symptoms and quality of life.
Oculomotor Nerve
An isolated oculomotor nerve palsy (CN III; Figure 1) presents a multifaceted challenge in both diagnosis and treatment. Understanding the nerve’s anatomy, as well as the symptoms and etiology of a CN III palsy, is crucial for effective diagnosis, treatment and management.
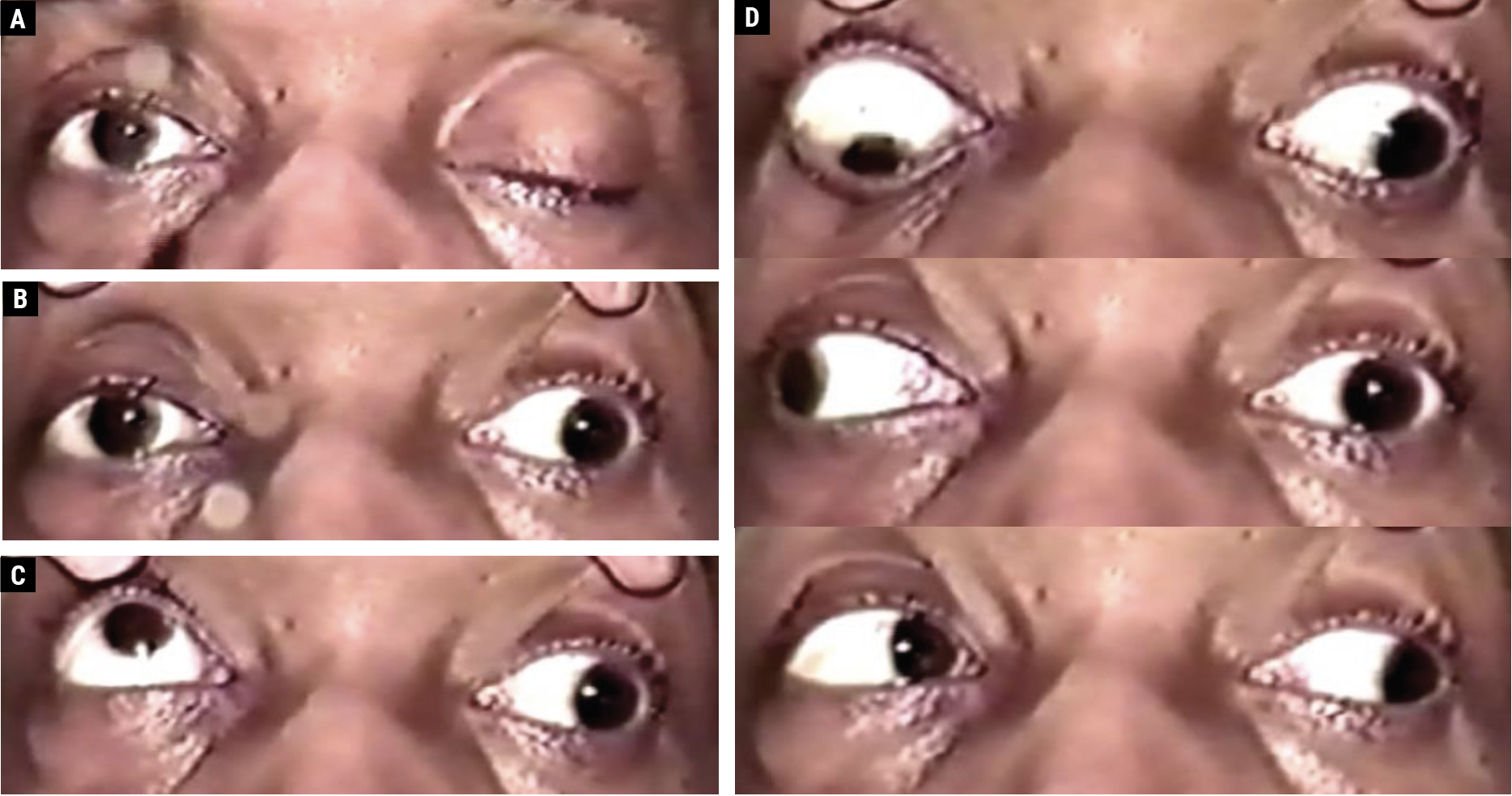 |
|
Fig. 1. A 48-year-old male with an aneurysmal left CN III palsy with complete upper eyelid ptosis (A). With manual eyelid elevation, the left eye is positioned out and slightly down; the left pupil is mid-dilated and reacts poorly to a light stimulus (B). On attempted upgaze, the right eye elevates fully, and there is a supraduction deficit involving the left eye (C). The righthand images (D) depict the following (top to bottom): on attempted downgaze, the right eye depresses fully, and there is an infraduction deficit involving the left eye; on attempted right gaze, the right eye abducts fully, while an adduction deficit involves the left eye; on attempted left gaze, there is complete adduction of the right eye and complete abduction of the left eye, which remains slightly depressed. Click image to enlarge. |
The oculomotor nerve originates from its nucleus in the dorsal midbrain at the level of the cerebral peduncles. It exits at the interpeduncular fossa and travels anteriorly between the posterior cerebral artery and the superior cerebellar artery, then runs alongside the posterior communicating artery.1 It travels in the lateral wall of the cavernous sinus above the trochlear nerve as it approaches the superior orbital fissure.
Next, the oculomotor nerve splits into two main divisions: the superior division and the inferior division. The former controls the superior rectus and levator palpebrae superioris muscles, while the latter governs the inferior rectus, inferior oblique and medial rectus muscles. The inferior division also includes parasympathetic fibers, which are responsible for pupil constriction and ciliary muscle contraction.2,3 A third nerve palsy can arise from damage at any point along this pathway.
Patient presentation can vary depending on which fibers of the oculomotor nerve are affected. If the superior division of CN III is involved, it may result in difficulty looking upward and a ptosis. If the inferior division is involved, the patient may have difficulty with adduction and downward gaze. A complete CN III palsy leads to reduced elevation, depression and adduction with a significant ptosis. In this case, the eye may present with the classic “down and out” appearance once the ptotic lid is elevated. An incomplete palsy will have partial limitation of these eye movements or a less significant ptosis. Regardless of whether the palsy is complete or incomplete, the pupil presentation can vary. In addition to the classic “blown” pupil, it may present as a more subtle anisocoria in bright illumination. In some cases, the pupil of the affected eye might only be sluggishly reactive, indicating partial pupillary fiber involvement.4
Patients with isolated CN III palsy may experience a range of symptoms. Often these patient present with horizontal and vertical diplopia at both distance and near.5 With a complete palsy, cover test typically displays a hyper-deviation that increases in magnitude in upgaze and reverses in downgaze. In addition, there will be an exo-deviation greater at near than at distance and when looking in the opposite direction of the affected eye.
The etiology of an isolated CN III palsy is diverse and can range from benign to life-threatening. Key considerations include pupil involvement, microvascular history and history of trauma. While it was previously believed that pain could indicate an aneurysmal cause, we can no longer rely on pain as a definitive indicator of etiology. Approximately 60% of individuals report experiencing pain or headaches, but this symptom does not differentiate between aneurysmal and ischemic origins.6 More commonly, ischemic microvascular changes from diabetes, hypertension or hyperlipidemia can infarct the vasa nervorum, the blood vessels that nourish CN III and lead to palsy.7 Non-ischemic pathologies include neoplasm, aneurysm, demyelinating disease, trauma, cavernous sinus masses or malformations and giant cell arteritis.8
If the pupil is involved, an aneurysm, particularly of the posterior communicating artery, should be suspected. Other locations to suspect an aneurysm include the internal carotid artery (ICA), basilar artery or anterior communicating arteries. Occasionally, patients with a CN III palsy caused by aneurysmal compression present with an incomplete palsy without pupillary involvement. Consequently, a patient presenting with an incomplete palsy (even without pupil involvement) is just as concerning as one presenting with pupillary involvement. Both constitute emergencies that demand immediate medical attention.9
In cases of isolated CN III palsies without pupil involvement, an ischemic vascular etiology should be considered. Common causes of ischemic vascular CN III palsies include diabetes, hypertension and giant cell arteritis. Diabetic and hypertensive workups should be conducted, as well as laboratory studies, including a complete blood count, erythrocyte sedimentation rate and C-reactive protein levels.10 Imaging with MRI, MRA or CT angiography within one week is advised, as 16.5% of patients with CN III palsy have an etiology other than presumed microvascular disease.10 If the CN III palsy is suspected to be aneurysmal, it is a medical emergency that necessitates immediate MRI, MRA or CT angiography. The patient should be promptly referred to the closest hospital emergency department (ED), and the referring physician should communicate directly with the ED physician. In cases of aneurysm, the risk of rupture, subarachnoid hemorrhage and death is elevated, making immediate neurosurgical intervention essential. Typical treatments for aneurysmal CN III palsies include embolization with coils or direct clipping of the aneurysm.11
Management of a CN III palsy requires addressing the etiology. Regardless of a patient’s vascular risk factors, neuroimaging and relevant lab tests play a crucial role in evaluating patients with an isolated CN III palsy.12
While ischemic vascular palsies typically improve spontaneously within three to six months of initial presentation, if there is no improvement, repeated neuroimaging is necessary. Monitoring on a monthly basis is advised to assess improvement, stability or progression. Acute diplopia can be managed with occlusion patches and Fresnel prisms to address residual double vision. Extraocular motility deviations that have been stable for 12 months may benefit from strabismus surgery and ptosis surgery.13
Aberrant regeneration often occurs as the nerve heals the internal damage that led to the palsy. Aberrant regeneration occurs when nerve fibers misdirect their growth after injury. Aberrant regeneration is most associated with aneurysms, tumors and trauma but is rare with ischemic vascular conditions like diabetes or hypertension. Classic signs of aberrant regeneration include light near dissociation when the pupil constricts with adduction due to misdirection of fibers to the iris sphincter. Another example is eyelid synkinesis when the eyelid elevates upon adduction due to medial rectus fibers misdirecting to the levator palpebrae superioris. Pseudo-Graefe’s sign occurs when the upper eyelid elevates as the patient looks down, due to the inferior rectus fibers misdirecting to the levator palpebrae superioris.14
Isolated CN III palsy is a complex condition with various potential causes and implications. Mimickers of a CN III palsy can include internuclear ophthalmoplegia, myasthenia gravis and thyroid eye disease (TED). To avoid misdiagnosis, a thorough understanding of its anatomy and the symptoms and etiologies of CN III palsies is essential.15
Trochlear Nerve
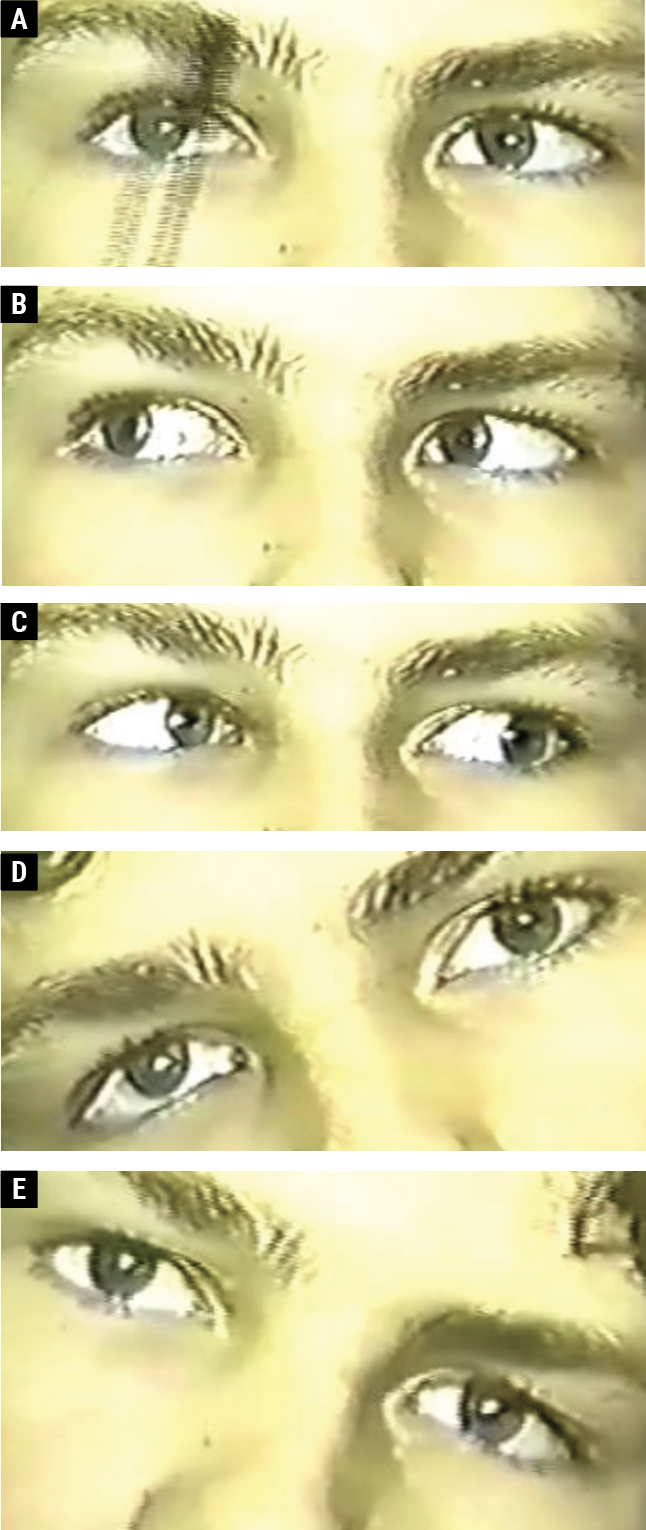 |
| Fig. 2. A 13-year-old male with a right CN IV palsy. The right eye is hyper in primary gaze (A). There is vertical orthophoria in right gaze (B); right hypertropia on left gaze (C); right hypertropia on right head tilt (D); and vertical orthophoria on left head tilt (E). Click image to enlarge. |
Patients with trochlear nerve (CN IV; Figure 2) palsy typically experience vertical diplopia, especially with near work. There may be an inability to look down and in. Relief is often found by tilting the head away from the affected side and tilting the chin down.16 An alternate cover test will reveal hypertropia, which worsens on ipsilateral head tilt and upon contralateral gaze.17
Common etiologies of CN IV palsies include congenital maldevelopment, trauma and ischemic vascular disease, the latter being especially common in adults over 40.18 Blunt head trauma can lead to isolated unilateral or bilateral trochlear nerve palsies. Other causes include schwannomas, metastatic tumors, vascular malformations and demyelinating disease.19
The CN IV is particularly vulnerable to injury or compression due to its unique anatomical path; as the thinnest CN with the longest intracranial pathway, it is susceptible to various forms of mechanical injury along its course. It originates from its nucleus at the level of the inferior colliculus where the fascicles exit the dorsal midbrain and cross. After the decussation, the nerve travels anteriorly between the posterior cerebral artery and superior cerebellar arteries within the ambient cistern of the subarachnoid space. The nerve continues to pass through the lateral wall of the cavernous sinus and traverses the superior orbital fissure to ultimately innervate the superior oblique muscle.12 The superior oblique aids in depression, intorsion and abduction.
Tests to assist with diagnosis of CN IV palsy include the Parks three-step test and examination of the degree of excyclotorsion. Parks three-step test identifies which eye is higher in primary gaze, which eye shows more hyper-deviation in right or left gaze and which eye shows more hyper-deviation when tilting the head.20 Bilateral CN IV palsies may be difficult to diagnose and should be suspected with alternating hypertropia worse in contralateral gaze and ipsilateral head tilts with more than 10° of objective excyclotorsion bilaterally. These examples emphasize the importance of performing cover test or single Maddox rod in all nine gazes and head tilt. Examining excyclotorsion can be evaluated subjectively with double Maddox rods or objectively by examining the degree of excyclotorsion from fundus photos or OCT.
Differentiating between a decompensated congenital nerve palsy and an acquired CN IV palsy is crucial. Congenital cases often result from agenesis or anomalous muscle/tendon insertion and are characterized by large vertical prism fusional amplitudes, allowing compensation with a head tilt. Reviewing old photographs can help distinguish congenital conditions from recently acquired ones. Facial microsomia contralateral to the trochlear palsy is indicative of a congenital cause. Hypertropia that is more pronounced in upgaze is indicative of congenital or decompensated palsies, distinguishing them from other types where hypertropia may be worse in downgaze.21
For both children and adults with longstanding unilateral CN IV palsies accompanied by a history of either trauma or increased vertical vergences suggesting a congenital etiology, the traditional approach has been observation. However, neuroimaging is now recommended for all patients with cranial nerve IV involvement to identify and evaluate potential underlying conditions. In cases of bilateral CN IV palsy, suspected dorsal midbrain syndrome, involvement of other cranial nerves, absent history of trauma or a suspected acute ischemic event, an MRI is recommended to provide a clearer diagnosis.22
Management strategies vary based on the underlying cause. Ischemic causes generally show improvement within three to six months after initial presentation, with monthly follow-ups recommended to monitor improvement. Workup is warranted if progressive worsening occurs rather than improvement. Traumatic causes also tend to improve within six to 12 months and should be monitored regularly for improvement. Occlusion therapy or base-down prism can be employed to minimize diplopia.23
Surgery may be considered only if there is no improvement after about 12 months. Strabismus surgery is an option for persistent, longstanding trochlear nerve palsies that are not amenable to other treatments.24 Other differentials that mimic CN IV palsy can include orbital pseudo tumor/orbital mass, myasthenia gravis, skew deviations and TED.
Abducens Nerve
The lateral gaze centers, which include the abducens nucleus and the paramedian pontine reticular formation on both sides, are essential for lateral eye movements. They coordinate innervation of the ipsilateral CN VI nucleus to control the lateral rectus muscle and the contralateral medial rectus muscle via CN III, using the medial longitudinal fasciculus for communication.
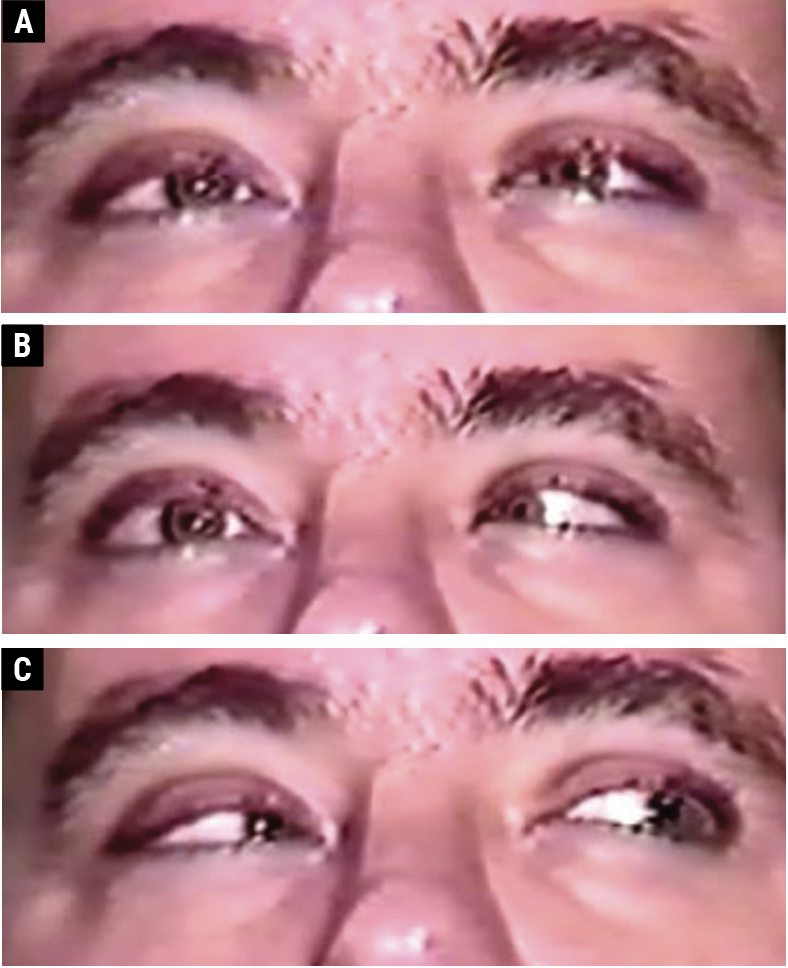 |
| Fig. 3. A 36-year-old male with an ischemic vascular right CN VI palsy. There is orthophoria in primary gaze (A); a right abduction deficit in right gaze (B); and orthophoria in left gaze (C). Click image to enlarge. |
The CN VI pathway begins within the abducens nuclei housed in the low pons, located near the facial colliculus. Axons exit the brainstem at the pontomedullary junction passing between the anterior inferior cerebellar artery and internal auditory artery. From there, the nerve ascends along the clivus and crosses the petrous portion of the temporal bone and travels through Dorello’s canal to enter the cavernous sinus, within which CN VI travels more medial than the other cranial nerves and is lateral to the ICA. It then passes through the superior orbital fissure to innervate the lateral rectus.20
CN VI palsies (Figure 3) typically present with horizontal diplopia, worse at distance than at near. Patients often turn their faces toward the side of the palsy to minimize double vision; for instance, a left-sided CN VI palsy will compensate with their face turned to the left. The condition is characterized by an abduction deficit and esotropia worsening in the field of action of the paretic muscle. Patients may also exhibit slowed horizontal saccadic movements or glissades.25
When diagnosing a CN VI palsy, it is essential to differentiate it from conditions such as TED, myasthenia gravis and Duane’s retraction syndrome, particularly type I. Forced duction testing can help in diagnosis; if the eye can be moved without resistance, the test is negative for a restrictive process and could indicate CN VI palsy or myasthenia gravis, as two examples. Conversely, if the eye resists movement, the test is positive, indicating restricted movement from conditions such as TED.26
Conditions affecting the petrous temporal bone or subarachnoid space can lead to CN VI palsy. Elevated intracranial pressure can cause papilledema and induce this type of palsy by compressing the nerve against the petrous temporal bone. Gradenigo’s syndrome, related to mastoiditis, may present with painful CN VI palsy, hearing issues, ear discharge and periorbital or retro-orbital pain. Other potential causes include aneurysm, ischemic vascular disease, trauma, meningitis, clivus or supratentorial tumors, multiple sclerosis or infections.27
Neuroimaging is recommended for all patients with CN VI palsy. In children, potential etiologies include neoplasm, infection, inflammation and idiopathic etiologies. For young adults, multiple sclerosis is a prevalent cause of CN VI palsy.27 For ischemic cases, recovery typically occurs within three to six months and should be monitored monthly for improvement. Compressive lesions generally worsen over time, so worsening of a suspected ischemic CN VI should be re-imaged. Accurate diagnosis and appropriate management are crucial for effective treatment and recovery from abducens nerve palsy.
Cavernous Sinus
In the case of a cavernous sinus mass or malformation, multiple CNs, including CN III, IV, V and VI, as well as pupil function, can be affected. Differential diagnoses for such presentations include cavernous sinus masses, fistulas and Tolosa-Hunt syndrome. Cavernous sinus masses, like meningiomas or pituitary adenomas, can grow laterally into the cavernous sinus compressing these cranial nerves and causing dysfunction. A cavernous sinus fistula is an abnormal connection between the arterial and venous systems within the sinus. Tolosa-Hunt syndrome, an idiopathic inflammatory condition, typically presents with acute onset of CN palsies accompanied by pain. A thorough examination of all CNs (I to XII) is crucial when a patient presents with even a single CN palsy.28
Takeaways
Cranial nerve palsies involving the oculomotor (CN III), trochlear (CN IV) and abducens (CN VI) nerves present significant challenges in diagnosis and management due to their varied etiologies and clinical manifestations. Each type of palsy is associated with distinct symptoms, such as diplopia and specific limitations in eye movement, influenced by the anatomical pathways of the nerves and potential underlying conditions.
When dealing with any cranial nerve palsy, current guidelines emphasize the importance of neuroimaging to rule out serious underlying causes. Accurate diagnosis and timely intervention are essential for effective management and improved patient outcomes. By understanding the complexities of these cranial nerve palsies and implementing appropriate follow-up strategies, healthcare providers can significantly enhance the recovery and quality of life of those affected.
Dr. Messner is the vice president for strategy and institutional advancement at Illinois College of Optometry (ICO), where he holds the rank of Professor of Optometry. He is a fellow of the American Academy of Optometry (AAO), the immediate past chair of the AAO’s Neuro-ophthalmic Disorders Special Interest Group and a member of the AAO’s steering committee of the Fellows Doing Research Special Interest Group. Dr. Messner has received various optometric teaching awards and was recognized as Optometrist of the Year in Illinois in 2013. He is on the advisory board of the Concussion Legacy Foundation, Heidelberg Engineering and Horizon Therapeutics. Dr. Piraino is a 2024 graduate of ICO, where she is currently in residency training for neuro-optometry. Dr. Stone is the assistant dean for didactic education. She joined the ICO faculty full-time in 2005, before which she worked in private practice. She sees patients mainly in the primary care department and also co-teaches the neuro-ophthalmic disorders course. Dr. Stone is a fellow of the AAO and a consultant for the Accreditation Council on Optometric Education.
1. Martins C, Yasuda A, Campero A, Rhoton AL Jr. Microsurgical anatomy of the oculomotor cistern. Neurosurgery. 2006;58(4 Suppl 2):ONS-220-7; discussion ONS-7-8. 2. Park HK, Rha HK, Lee w, Chough CK, Joo W. Microsurgical anatomy of the oculomotor nerve. Clin Anat. 2017;30:21-31. 3. Sanders M. Walsh and Hoyt’s clinical neuro-ophthalmology. J Neurol Neurosurg Psychiatry. 1996;61:236-7. 4. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;1;135(1):23-8. 5. Yanovitch T, Buckley E. Diagnosis and management of third nerve palsy. Curr Opin Ophthalmol. 2007;18(5):373-8. 6. Tamhankar MA, Biousse V, Ying GS, et al. Isolated third, fourth and sixth cranial nerve palsies from presumed microvascular versus other causes: a prospective study. Ophthalmology. 2013;120(11):2264-9. 7. Jacobson DM, McCanna TD, Layde PM. Risk factors for ischemic ocular motor nerve palsies. Arch Ophthalmol. 1994;112:961-6. 8. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-68. 9. Kim JY, Choi SC. Third cranial nerve palsy and posterior communicating artery aneurysm. Clin Exp Emerg Med. 2014;1(1):65-6. 10. Tamhankar MA, Biousse V, Ying GS, et al. Isolated third, fourth and sixth cranial nerve palsies from presumed microvascular versus other causes: a prospective study. Ophthalmology. 2013;120(11):2264-9. 11. Gaberel T, Borha A, di Palma C, Emery E. Clipping versus coiling in the management of posterior communicating artery aneurysms with third nerve palsy: a systematic review and meta-analysis. World Neurosurg. 2016;87:498-506.e4. 12. Shree R, et al. Oculomotor cranial neuropathies: diagnosis and management. Ann Indian Acad Neurol. 2022;25(Suppl 2):S70-82. 13. Modi P, Arsiwalla T. Cranial nerve III palsy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2024. 14. Weber ED, Newman SA. Aberrant regeneration of the oculomotor nerve: implications for neurosurgeons. Neurosurg Focus. 2007;23(5):E14. 15. Schroeder RM, Stunkel L, Gowder MTA, et al. Misdiagnosis of third nerve palsy. J Neuroophthalmol. 2022;1;42(1):121-5. 16. Akagi T, Miyamoto K, Kashii S, et al. Cause and prognosis of neurologically isolated third, fourth, or sixth cranial nerve dysfunction in cases of oculomotor palsy. Jpn J Ophthalmol. 2008;52(1):32-5. 17. Gräf M, Krzizok T, Kaufmann H. Head-tilt test in unilateral and symmetric bilateral acquired trochlear nerve palsy. Klin Monbl Augenheilkd. 2005;222(2):142-9. 18. Koller HP, Olitsky SE, O’Hara M, Nelson LB. Diagnosis and treatment of fourth nerve palsy. J Pediatr Ophthalmol Strabismus. 2016;53(2):70-4. 19. Mielke C, Alexander MS, Anand N. Isolated bilateral trochlear nerve palsy as the first clinical sign of a metastatic bronchial carcinoma. Am J Ophthalmol. 2001;132(4):593-4. 20. Kumar A. Parks 3-step test. Indian J Ophthalmol. 2022;70(8):3167. 21. Yang HK, et al. Congenital superior oblique palsy and trochlear nerve absence. Ophthalmology. 2012;119(1):170-7. 22. Lyons CJ, et al. Cranial nerve palsies in childhood. Eye. 2015;9;29(2):246-51. 23. Campellone J, Ed. Fourth nerve plasy. Cedars Sinai. 2022. 24. Khanam S, Sood G. Trochlear nerve palsy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2024. 25. Patel SV, Mutyala S, Leske DA, Hodge DO, Holmes JM. Incidence, associations and evaluation of sixth nerve palsy using a population-based method. Ophthalmology. 2004;111(2):369-75. 26. Graham C, Gurnani B, Mohseni M. Abducens nerve palsy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2024. 27. Hörner R, Kassubek J, Dreyhaupt J, Ludolph AC. The spectrum and differential diagnosis of acquired ocular motor nerve palsies: a clinical study of 502 patients. J Neurol. 2022;269(4):2140-8. 28. Kuybu O, Dossani RH. Cavernous sinus syndromes. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2024. |

