Clinical Research Crash CourseMedical statistics are only meaningful if you know how they’re calculated and why. To help ODs feel more confident interpreting clinical study findings and stats, Review of Optometry published a four-part series on scientific research and how it relates to clinical practice. Browse the features in this series:
|
Research studies are the lifeblood of medical practice. They answer important questions and guide a practitioner through the delivery of care. Optometrists need to keep dozens of significant studies in their heads at all times and know how to apply them to the patient in the chair. With the constant influx of data from the research community, it can be challenging for ODs to discern when and how to integrate new findings into their clinical practice. Adopting new protocols too quickly might result in unintended consequences, while delay could mean missing opportunities to improve patient outcomes.
Brian Chou, OD, who practices in San Diego at ReVision Optometry, a referral-based clinic for keratoconus and scleral lenses, acknowledges the complexities of integrating medical research into daily practice. He notes that although many healthcare practitioners claim to be evidence-based, “this proclamation is sometimes inconsistent with what they actually do.” For instance, many practitioners continue to routinely prescribe 5% NaCl ointment for recurrent corneal erosion, “even though good evidence suggests the hyperosmotic works no better than bland ointment,” he points out.
Download PDFClick here for a PDF version of this guide to 60 landmark studies in eye care.
|
In this article, part 3 of a series on the role of research in clinical practice, we will review a plethora of pivotal studies that have shaped the field of eye care. Reflecting the collective wisdom of over 20 optometric experts who contributed, this guide explains the key takeaways, practice implications and legacy of each study in today’s clinical landscape. Links to the journal articles are included for all studies mentioned here so that our readers can also access the primary sources if they wish.
Glaucoma
OHTS Ocular Hypertension Treatment Study. Optometrists have relied on this landmark work for over 20 years, as it clarified important components of how ocular hypertension may or may not progress to glaucoma.
Investigating the efficacy of topical ocular hypotensive meds in delaying or preventing the onset of primary open-angle glaucoma (POAG), OHTS explored several important questions around early treatment effects, baseline risk factors and the consequences of delaying treatment.
The analysis led to a shift in clinical care, offering optometrists guidance on intervention vs. close monitoring for patients with low-risk ocular hypertension, according to Brad Sutton, OD, a clinical professor at Indiana University School of Optometry and service chief of the Indianapolis Eye Care Center.
 |
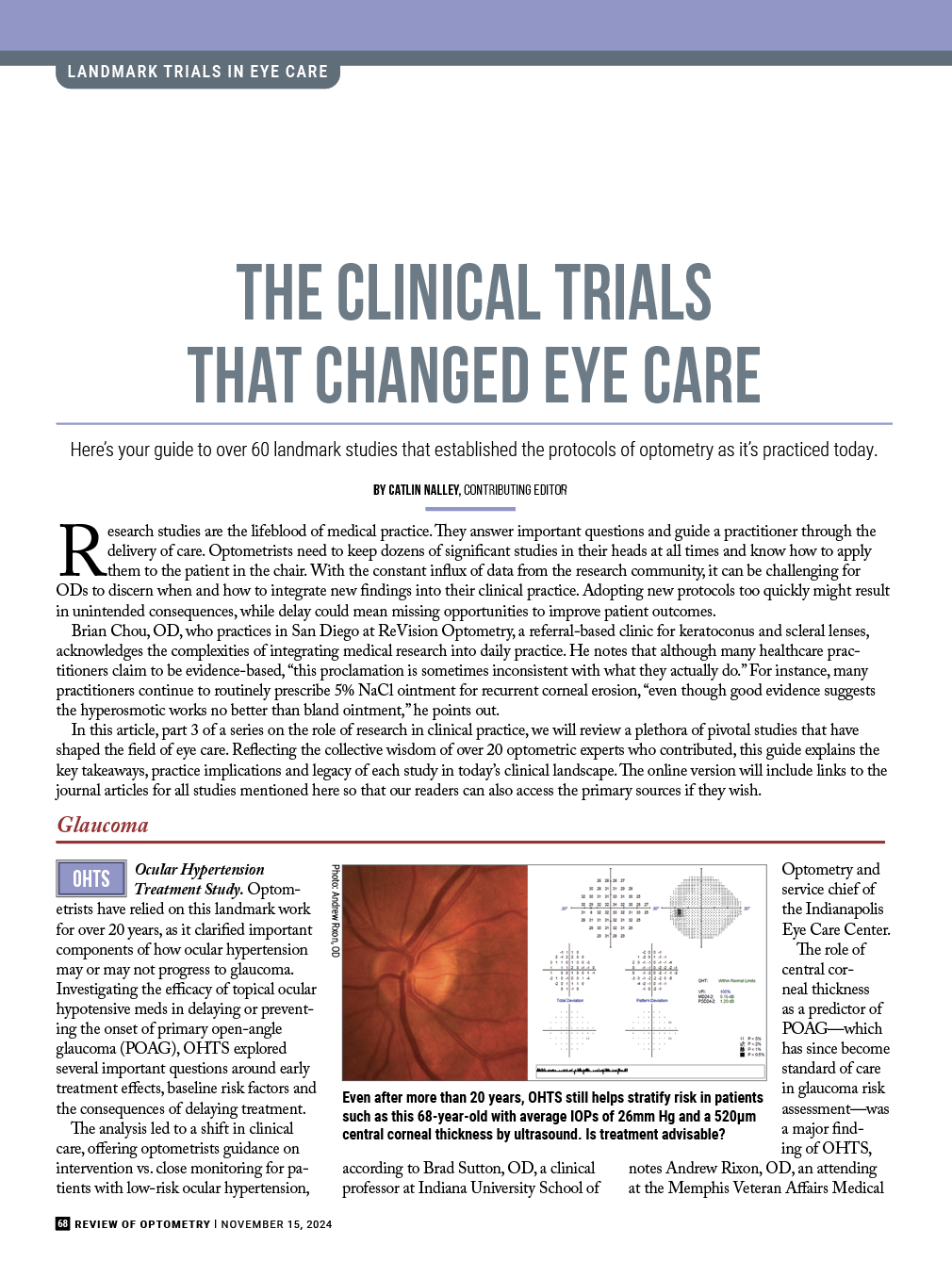
| Even after more than 20 years, OHTS still helps stratify risk in patients such as this 68-year-old with average IOPs of 26mm Hg and a 520µm central corneal thickness by ultrasound. Is treatment advisable? Photo: Andrew RIxon, OD. Click image to enlarge. |
The role of central corneal thickness as a predictor of POAG—which has since become standard of care in glaucoma risk assessment—was a major finding of OHTS, notes Andrew Rixon, OD, an attending at the Memphis Veteran Affairs Medical Center. “The results of OHTS reinforced that ocular hypertension does not warrant treatment in many cases and careful observation depending on risk stratification is reasonable in most cases,” he explains.
Joseph Sowka, OD, an attending optometric physician at Center for Sight/US Eye in Venice, FL, says that the study’s findings “validated clinical thoughts that about 10% of ocular hypertension patients progress to glaucoma over five years” while also identifying those at higher risk who may benefit from treatment.
Although the study does not support the treatment of all patients with elevated intraocular pressure (IOP), it reinforces the trend that lower IOP does reduce the risk of further losses, says Andrew Gurwood, OD, a professor of clinical sciences at Pennsylvania College of Optometry at Salus University.
OHTS remains relevant today, but Dr. Rixon notes that advancements since its publication, notably OCT, have surpassed the circumstances of the original study’s methods. A similar analysis using current technology, such as OCT and corneal hysteresis, would be an interesting avenue for future research that could further refine risk prediction models, he suggests.
CIGTS Collaborative Initial Glaucoma Treatment Study. Which is better: meds or scalpels? It seems an easy choice, especially for new patients. And yet CIGTS, designed to compare the long-term effects of medical therapy vs. surgical intervention for newly diagnosed open-angle glaucoma (OAG), questioned the conventional wisdom circa 1999 of managing the entire patient population with eye drops.
Both treatment cohorts experienced significant and sustained reductions in intraocular pressure from baseline, with surgical patients maintaining IOP levels approximately two to three points lower than those treated with topical drugs, according to Dr. Gurwood. Patients in the trabeculectomy group initially experienced greater visual field loss and more acuity decline during the first three years; however, he notes, these differences largely diminished in years four and five.
Findings from CIGTS did not support a shift away from the standard medication-driven approach for newly diagnosed open-angle glaucoma. Dr. Sutton shares that “we still consider trabeculectomy to carry too much risk to be a first-line treatment,” reflecting a more cautious management approach.
Dr. Sowka points out that there are more patient-friendly procedures available today vs. 25 years ago, as minimally invasive glaucoma surgery (MIGS) and selective laser trabeculectomy (SLT) have created a middle category between meds and trabeculectomy.
Looking ahead, Dr. Sutton suggests that future research efforts could explore similar studies using modern glaucoma drugs. The optimal treatment approach for newly diagnosed OAG continues to evolve, and CIGTS was notable for breaking the dominance of initial medical therapy, even if its findings no longer reflect the current medical and surgical landscape.
EMGT Early Manifest Glaucoma Trial. The first large randomized controlled trial to assess the impact of IOP lowering on the progression of newly diagnosed open-angle glaucoma, EMGT was unique in that it compared the effect of immediate intervention vs. late or no treatment (as long as progression was not seen). Researchers observed considerable beneficial effects of treatment that significantly delayed disease progression.
“It provided clear evidence supporting the lowering of intraocular pressure in patients diagnosed with open-angle glaucoma and also added defense to what intuitive clinicians have asserted for years: pressure matters,” according to Dr. Gurwood, who previously discussed this and other optometric research in the journal Optometry; some of those comments have been cited and updated here in collaboration with him.1
One significant clinical implication of EMGT was the recognition that disease progression can vary among patients. “Some treated patients progress quickly, and some untreated patients remain stable,” says Dr. Sowka, while also noting that this supports monitoring for certain early glaucoma patients.
A more recent analysis, using data from the original EMGT study, delved deeper into the long-term impact of immediate vs. delaying treatment until progression occurred in early glaucoma and found that “with frequent and careful follow-up and frequent standardized functional testing, delaying treatment did not result in statistically significant differences in serious penalties in terms of glaucoma-induced low vision and blindness between the two groups of subjects, with immediate treatment and no initial treatment.”2
For the control group, the percentages of eyes with blindness or visual impairment was counterintuitively slightly less than in the treatment group, the study authors report, while highlighting that these findings suggest that delaying the diagnosis and treatment of early-stage glaucoma by a few years may not result in significant visual function loss, though the study did show a slightly greater overall risk of field loss when patients were not initially treated.2
Dr. Sowka acknowledges that “few clinicians feel comfortable not treating diagnosed glaucoma.” This underscores the ongoing debate surrounding the balance between monitoring and treating early glaucoma, reflecting the lasting impact of this trial on clinical decision-making.
AGIS Advanced Glaucoma Intervention Study. A cornerstone of our understanding around the long-term outcomes of surgical intervention for advanced glaucoma, AGIS looked to clarify the effectiveness of various treatment sequences involving trabeculectomy and argon laser trabeculoplasty among patients who did not respond to medical therapy.
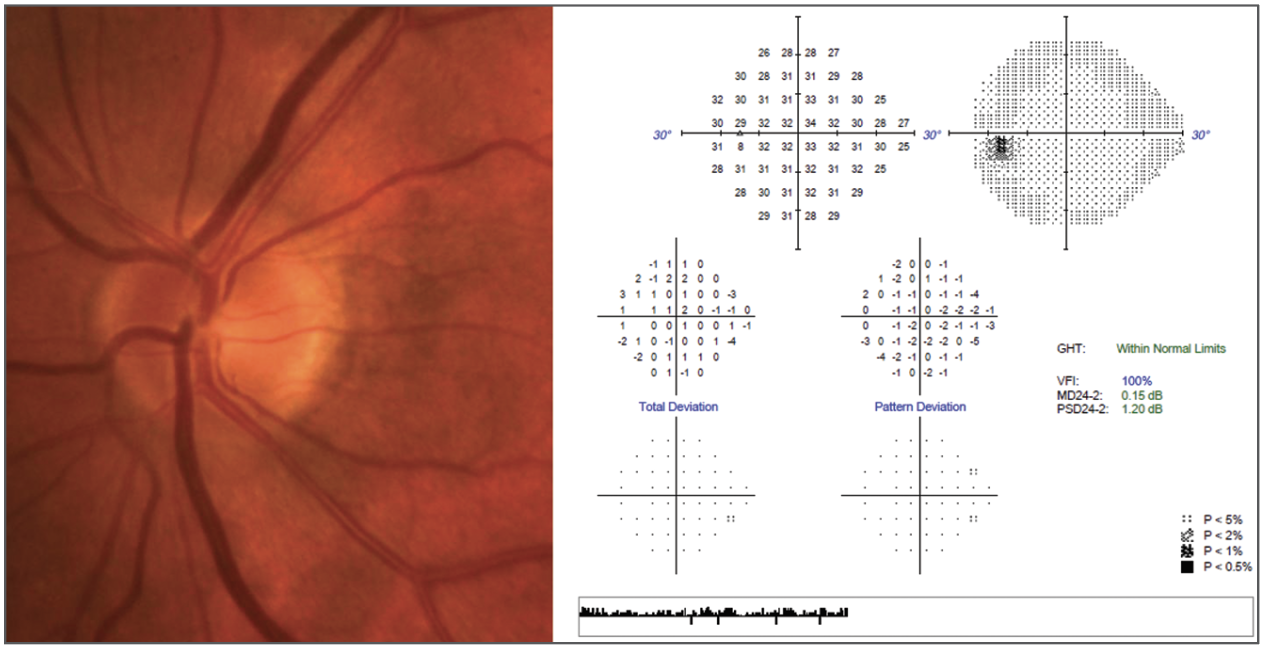
 |
| Do the findings of AGIS still guide us in the appropriate care of this 65-year-old patient of African descent with advanced disease? Photo: Andrew Rixon, OD. Click image to enlarge. |
“AGIS showed that substantially lowering IOP was associated with a reduction of visual field progression and that when patients achieving a lower mean IOP still continued to progress, that progression was associated with IOP fluctuation,” Dr. Rixon highlights.
The AGIS data also uncovered racial differences in treatment efficacy, with Black patients benefitting more from treatment starting with laser surgery, Dr. Gurwood notes. In comparison, while white patients initially benefited more from laser surgery, they demonstrated better outcomes with trabeculectomy over time. Based on these results, it was historically recommended that Black patients with advanced glaucoma (where topical therapy is failing) should begin treatment with laser surgery while their white counterparts would benefit from trabeculectomy first, if they have no life-threatening health problems.1
These conclusions were based on the assumption that genetic differences between white and Black individuals were able to explain the difference between the success of the respective surgeries when stratified by “race.” The current perspective is that race is a social construct and is not as genetically meaningful. Accordingly, glaucoma intervention should be personalized based on the patient’s clinical characteristics, not their self-reported race.3,4
The AGIS results have significantly influenced optometric care, establishing the importance of aggressive IOP reduction as a standard practice in glaucoma management. However, Dr. Sowka advises taking a more nuanced view, noting that the study’s findings have been misrepresented in the past, often by industry-aligned sources. He clarifies that while patients with consistently low IOPs (average 12mm) in AGIS showed minimal progression, the notion that an IOP below 18mm guarantees stability is misleading. “Too many believe that if IOP is less than 18mm, patients won’t get worse, and this creates a false sense of security about an incorrect target IOP.”
While the knowledge gained from this analysis still has a place in clinical practice today, Dr. Rixon notes that the surgical methods from AGIS are no longer applicable. Newer techniques, such as SLT and trabeculectomy with antifibrotic agents, have replaced the more tissue-damaging argon laser procedure and trabeculectomy sans antifibrotics.
“Future research could employ newer surgical technologies, use OCT as an endpoint and assess quality-of-life metrics,” he suggests, while pointing to the Treatment of Advanced Glaucoma Study (TAGS), which recently compared medical management with primary trabeculectomy augmented with mitomycin-C.5 “TAGS found primary trabeculectomy to be more effective at lowering IOP and slowing VF loss at five years when compared to primary medical treatment.”
CNTGS Collaborative Normal-Tension Glaucoma Study. If all our medical and surgical interventions are oriented around lowering IOP, where does that leave us in cases of normal-tension glaucoma? CNTGS examined the effectiveness of IOP reduction among such patients, a less studied and somewhat more mysterious entity than POAG.
Its data revealed that lowering an already normal IOP was indeed beneficial in slowing glaucomatous progression, says Dr. Sutton, who also notes that CNTGS justified the treatment of low IOP. Insights derived from this study challenged previous assumptions and highlighted the potential value of reducing IOP even in patients with normal baseline pressures.
Opinions vary on the current relevance of this study. Dr. Sutton believes the results are pertinent today, while Dr. Sowka has reservations, sharing that “the treatments used were very limited and the patient numbers were low.” Although the study’s conclusions are important, they may not fully reflect today’s clinical landscape, he says.
Dr. Rixon points out that CNTGS allowed at least one subject with IOP of 24mm Hg. “It’s difficult in the modern landscape with the knowledge that IOP is a 24/7/365 thing” to truly consider this a normal-tension study. “We would benefit from a study that only included pressures under 18mm Hg.”
To build on CNTGS’s foundation, Dr. Sutton recommends repeating the study with modern glaucoma drugs. This could offer more thorough insights and potentially lead to improved treatment strategies for patients with normal-tension glaucoma.
GLT Glaucoma Laser Trial. One of the oldest trials in our round-up, this 1990 study positioned argon laser trabeculoplasty (ALT) as a viable initial treatment option for POAG patients, offering an alternative to traditional medication-based approaches.
GLT compared ALT to topical meds and found that patients who underwent the laser procedure as their initial treatment had lower pressure as well as better visual field and optic disc status compared with those who started with medications. Dr. Sowka notes that ALT was “at least as effective as medicines,” and supported the procedure’s use as a first-line management option.
While this study shaped clinical practices at the time, its relevance has waned, according to Dr. Sowka, who notes, “ALT is no longer used in reasonable practice.”
However, the GLT did introduce what was to eventually become a sea change in the initial treatment of open-angle glaucoma: the notion that a laser procedure might be as good as or better than medical therapy. This notion is now emerging as the present-day standard of care thanks to the LiGHT study, discussed below.
LiGHT Laser in Glaucoma and Ocular Hypertension Trial. With the 2019 publication of the LiGHT trial, SLT really came into its own as a safe, effective and economical alternative to eye drops, supporting a shift in first-line treatment paradigms for glaucoma and ocular hypertension.
 |
|
The LiGHT trial solidified SLT as a viable first-line option for newly diagnosed open-angle glaucoma patients—so much so that the UK adopted this as a preferred course for clinicians. Photo: Nate Lighthizer, OD. Click image to enlarge. |
Addressing several important questions regarding the procedure’s viability compared to traditional medication-based therapies, the LiGHT trial showed a similar pressure lowering response between SLT and prostaglandin analog therapy—approximately 30%—according to Joseph Shovlin, OD, a senior optometrist at Northeastern Eye Institute in Scranton, PA, who notes that this validates the benefits of this approach.
Since the LiGHT trial, there has been a significant change in clinical practices. Remarking on its impact, Dr. Rixon notes that the study’s results led to the UK’s National Institute for Health and Care Excellence adopting SLT as the preferred first-line treatment for POAG. Additionally, the American Academy of Ophthalmology now includes the procedure as an acceptable first-line treatment for these patients. “Ultimately, first-line SLT has risen become to a widely accepted first-line option for those who are appropriate candidates, potentially offering a lifetime of drop-free care,” he emphasizes.
The findings from this analysis remain valuable today, with recently published six-year data further strengthening the case for SLT as a first-line treatment.6 Seventy percent of SLT recipients are still drop-free at six years, Dr. Shovlin reports.
There are a number of potential research avenues that could shed additional light on SLT and its role in optometric practice. For instance, Dr. Sutton suggests comparing new direct SLT, which does not require gonioscopy, to traditional SLT while Dr. Sowka advocates for real-world studies to better understand outcomes. “Personally, I don’t see SLT as effective as clinical studies do,” he notes.
Cornea
HEDS Herpetic Eye Disease Study. “This study was the first to show the benefit of prophylactic oral antivirals to decrease the recurrence rate of ocular herpes simplex manifestations,” notes Dr. Sutton. It evaluated the efficacy of topical corticosteroids in treating herpes simplex stromal keratitis in conjunction with topical trifluridine, as well as the efficacy of oral acyclovir in treating herpes simplex stromal keratitis and iridocyclitis, Dr. Gurwood explains.
 |
| The HEDS study ushered in an era of routine oral antiviral use in HSV keratitis patients to reduce risk of recurrence. Photo: Alison Bozung, OD. Click image to enlarge. |
This research established the importance of using topical steroids in herpetic stromal disease, according to Dr. Shovlin, who also notes that they found no benefit of oral prophylaxis to prevent progression from epithelial (infectious) to stromal (inflammatory) disease.
“Oral antivirals for one year significantly reduced the risk for recurrence of ocular herpes simplex, stromal keratitis (only in those who had a history of stromal disease) and oro-facial herpes simplex virus,” Dr. Shovlin adds.
The data resulted in major shifts in clinical care, especially regarding the use of prophylactic oral antivirals, which Dr. Sutton described as creating “a new treatment paradigm.” The impact of this research can still be seen today. Looking to the future, Dr. Sutton sees potential value in conducting a similar study that includes an arm with acyclovir, one with Famvir and one with Valtrex, while Dr. Shovlin points out that the Zoster Eye Disease Study (ZEDS) trial data, released at the American Academy of Ophthalmology 2024 annual meeting, revealed the effects of herpes zoster ophthalmicus (HZO) treatment on risk of herpes keratitis.
ZEDS found that one year of HZO treatment achieved a 26% reduction in the risk of new or worsening eye conditions (keratitis or iritis) at 18 months and a significant decrease in the number of flare-ups as well as pain symptoms.7
SCUT Steroids for Corneal Ulcers Trial. The best way to tamp down inflammation is with steroid therapy, but would doing so in a case of bacterial corneal ulcer expose the eye to any risks? SCUT explored this question and found that adding steroids “appropriately” was not harmful and was beneficial in cases involving the visual axis, Dr. Sutton advises.
Dr. Shovlin notes that “topical steroids offer no significant benefit (or risk) in treating bacterial keratitis” but emphasizes that steroids should not be used in Nocardia infections. The SCUT results also indicate that antibiotics with lower minimum inhibitory concentrations are associated with better outcomes, he reports. The 12-month results showed that although the benefits of using topical steroids may be delayed, there was no significant advantage observed after one year, Dr. Shovlin says. “There may be a benefit with adjunctive steroid use if application occurs early.”
When used wisely, the study suggests, steroids are an option for this patient population, according to Dr. Sutton. Dr. Sowka is less sanguine, noting that while steroids generally did not harm patients, they also did not significantly help them. Dr. Sowka also highlights what he considers the flawed nature of the study. “Final visual acuity was a major endpoint, and the majority of patients didn’t have a benefit from steroid use,” he elaborates. “However, the majority of patients had lesions off the visual axis and those would be expected to have the same visual outcome with or without steroids.” Dr. Shovlin points out that the High-Dose Steroid Study showed that aggressive steroid treatment (6+ drops per day started within seven days of corneal scraping) did yield better visual outcomes.8
To build on SCUT’s findings, Dr. Sutton proposes conducting a similar study with a stronger steroid while still ensuring the trial remains blinded. The original trial, he notes, used pred phosphate—a relatively weak steroid—because it is a clear solution that matched the placebo artificial tear.
CLEK Collaborative Longitudinal Evaluation of Keratoconus. This large observational study provided extensive insights into the natural history and progression of keratoconus.
 |
| CLEK highlighted eye rubbing’s impact on keratoconus development, among others. Photo: Getty Images. Click image to enlarge. |
Earlier studies of keratoconus primarily concentrated on its incidence, prevalence, causes or clinical management, and very few examined the clinical course and risk factors for progression in substantial sample sizes, notes Dr. Gurwood. In an effort to fill this knowledge gap, CLEK took a closer look at the clinical course of keratoconus as well as relationships among its visual and physiological manifestations. The study also sought to offer insights into risk and protective factors influencing its severity and progression.1
CLEK followed 1,209 patients over eight years, beginning in 1995, and generated a wealth of data that enhanced clinicians’ understanding of the condition, shares Dr. Chou. Notably, the data showed that 14% of participants had a family history of keratoconus and an unusually large number had a history of eye rubbing, allergies, asthma and atopic dermatitis. A 20% incidence of corneal scarring over the study period was also reported, according to Dr. Chou, more commonly in patients using flat-fitting rigid gas permeable (GP) lenses.
This data influenced many clinicians to minimize the prescribing of GP contacts with a flat-fitting relationship and to advise their patients against eye rubbing, Dr. Chou suggests, while emphasizing that this was based only on “CLEK-identified associations, not proven causation of disease progression.” The study also highlighted the value of disease screening for family members.
While it is important to realize that this study was completed prior to the 2016 FDA approval of corneal crosslinking and the widespread use of scleral lenses for keratoconus, its takeaways are still valuable. Dr. Chou notes, “The CLEK data supports concern for disease progression among younger keratoconus patients with elevated odds ratios for penetrating keratoplasty, corneal scarring and corneal steepening for the youngest keratoconus patients.”
Dr. Chou highlights the need for better characterization of the role of age in keratoconus progression, which could yield age-based recommendations for crosslinking. “More granular data in this area may spur our profession to incorporate topography as part of every routine eye exam. With CLEK, I suspect a larger number of keratoconus patients already had relatively stable disease by the time they received diagnosis,” Dr. Chou says. “Today, diagnosis is more sensitive with corneal topography and tomography, so with earlier diagnosis it’s possible that clinicians will observe greater progression.”
Dry Eye
OSDI Ocular Surface Disease Index. When Allergan was developing Restasis in the 1990s, it knew it needed a way to formalize the measurement of dry eye symptoms, a notoriously subjective and inconsistently reported experience. So, the company pursued the development and validation of a questionnaire for assessment of dry eye disease severity. The OSDI has been a mainstay of both research and clinical practice ever since, notes Andrew Pucker, OD, PhD, executive director of clinical and medical sciences at Lexitas Pharma Services.
 |
| The OSDI survey brought consistency to the measurement of dry eye symptoms. It’s routinely used in trials, as well as in practice. Photo: Allergan. Click image to enlarge. |
It is now used throughout most dry eye clinical trials as the gold standard of subjective questionnaires about dry eye disease and is in fact one of the few questionnaires accepted by the FDA for its psychometric properties, adds Natasha Hindman, OD, director of clinical research at Scott and Christie Eyecare Associates in the Pittsburgh area.
The relevance and clinical applicability of OSDI is still robust. Alongside other validated instruments, such as the SANDE, DEQ-5 and SPEED, it continues to be one of the best tools for tracking and diagnosing dry eye symptoms, Dr. Pucker says. A seven-unit change in the OSDI has been validated as clinically meaningful, noted Kelly Nichols, OD, dean of the University of Alabama at Birmingham School of Optometry, an educator renowned for her expertise in ocular surface disease.
While it would be challenging to develop, a questionnaire made directly by patients could be valuable, suggests Dr. Hindman while discussing future directions. “These questions are hard for patients to answer at times because they don’t see these things as being part of their symptoms. If you ask a patient what questions they think should be asked of their dry eye symptoms, they may give better questions for a future standardized test.”
Additional vision-related questions—about device use and blurry, changeable vision, for instance—as well as quality of life could be further investigated in any efforts to build upon the nearly 30-year-old OSDI questionnaire, Dr. Nichols adds.
DEWS TFOS DEWS I and TFOS DEWS II (additional links in sidebar below). These mammoth reports from Tear Film and Ocular Surface Society (TFOS) are essentially the bible of dry eye. Offering a comprehensive, evidence-based consensus on the definition, classification, diagnosis and management of dry eye disease to date at the time of each report, TFOS DEWS I and DEWS II have shaped most facets of how optometrists approach the condition.
“These white-paper style reports involved clinicians and researchers from across the world and used a very rigorous literature review and forward-looking statements approach,” explains Dr. Nichols, who also praises several complementary works from the same group: the TFOS Meibomian Gland Dysfunction report, the TFOS Contact Lens Discomfort report and the TFOS Lifestyle and Ocular Surface report.
This research is a “one-stop shop for all information relevant to dry eye,” and “helped bring uniformity to the dry eye community,” says Dr. Pucker. DEWS II in particular added to the understanding of this condition by incorporating neurological components and underscoring hyperosmolarity in the definition, notes Paul Karpecki, OD, director of cornea and external disease at the Kentucky Eye Institute in Lexington, KY. Also in DEWS II, “a new diagnostic algorithm attempted to streamline dry eye diagnosis, and a summary of existing treatments (at the time) was published,” notes Dr. Nichols. “The reports clarified that meibomian gland dysfunction (evaporative dry eye) and aqueous-deficient dry eye can occur simultaneously and have to both be managed appropriately.”
The significance of the studies is still present today. According to Dr. Pucker, principles of DEWS I and II should be the basis for every dry eye study, while Dr. Karpecki emphasizes its importance in clinical settings, noting that the DEWS II diagnostic algorithm is a “concise and effective means of dry eye diagnosis that also separates dry eye into evaporative and aqueous deficient.”
TFOS DEWS III, which will provide an updated definition and summarize the latest information since DEWS II, is expected to publish some time in 2025.
The TFOS Team Looks Back—and ForwardBy James S. Wolffsohn, PhD, Jennifer P. Craig, PhD, and Lyndon Jones, PhD The importance of dry eye as a clinical entity had been known at least since 1903, when Otto Schirmer devised his namesake test of lacrimal gland production. But despite its ubiquity among diverse populations worldwide, dry eye didn’t really garner much attention in research circles until about the mid-20th century; even then, results lacked a systematic approach. By the early 1990s, it was clear that a coordinated, interdisciplinary approach was needed to bring rigor to the field and so the fledgling Tear Film & Ocular Surface Society (TFOS) held its first conference, in Bermuda, in 1992. Those assembled identified gaps in informing dry eye clinical trials; three years later, the National Eye Institute published a workshop to define dry eye disease and discuss various aspects, such as appropriate clinical trial methodologies. By 2004, TFOS had begun building upon this initiative, which resulted in its first evidence-based consensus report, the 2007 TFOS Dry Eye Workshop (DEWS). Publication of TFOS DEWS highlighted the critical role meibomian gland dysfunction (MGD) plays in ocular surface disease and recognized a need to consolidate the research in this area to help guide future research priorities. This prompted TFOS to lead the International Workshop on MGD, which was published in 2010 and was followed by a Contact Lens Discomfort Workshop in 2013. A decade after the original report, TFOS DEWS II was published in 2017. This reviewed the exponentially increasing number of publications on the topic of dry eyes since the 2007 report, most importantly focusing on: • defining dry eye as a disease, not a syndrome, that is symptomatic (such that dry eye is a subset of ocular surface disease); • the concept of the homeostasis of the tear film being disrupted, with critical features within its pathophysiology that differentiates it from other ocular surface conditions; and • acknowledging that neurosensory factors can play a role. Moving away from the earlier trigger-focused approach, subclassification of the disease took a practical, patient-focused approach in TFOS DEWS II, with the presence of symptoms and signs leading to a patient being diagnosed with dry eye, another form of ocular surface disease (where signs are present without symptoms) or potentially neuropathic disease (where there are symptoms without accompanying clinical signs). The importance of differential diagnosis was articulated (with a view particularly to aiding practitioners beyond the eyecare professions who are involved in the “journey” of patients with dry eye disease) and a revised, more clinically applicable diagnostic algorithm was generated. Consistent with the definition, this diagnostic process seeks to identify symptoms which, together with key signs of a loss of homeostasis of the tear film, constitute a diagnosis of dry eye disease. In terms of disease management, it was noted that while high quality evidence demonstrating the efficacy of various treatments relative to a control exists, there was less evidence to aid clinicians in choosing the appropriate management strategy for patients with differing disease severity and subtypes (evaporative or aqueous deficient). The TFOS DEWS II reports have proved to be impactful both in research and clinically. They have been widely cited in other journal papers: the Definition and Classification report 3,168 times, Epidemiology 2,332 times, Diagnostic Methodology 1,882 times and Pathophysiology 1,672 times as of October 2024. These reports constitute six of the eight most downloaded articles from The Ocular Surface in the last 90 days (even seven years since their publication), and they form the basis of current recommendations of global professional organizations such as the World Council of Optometry’s Dry Eye Wheel and American Academy of Ophthalmology’s Preferred Practice Patterns. More recently, in 2023 TFOS published a special issue on the impact of lifestyle factors (including elective medications and procedures, contact lenses, lifestyle challenges, environmental conditions, societal challenges, cosmetics, the digital environment and nutrition) on the epidemic of ocular surface disease. These freely available TFOS Lifestyle reports highlighted just how much the modern environment and lifestyle choices are stressing the ocular surface and contributing to the increased prevalence of dry eye disease. As well as translations into various languages, clinically relevant outputs arising from the reports include informative infographics highlighting the key clinical outcomes available from the Centre for Ocular Research & Education in Canada. Almost another decade on from TFOS DEWS II, the volume of new literature in the field—over 9,000 additional peer reviewed articles—has prompted a third dry eye workshop, TFOS DEWS III, which is currently underway, reviewing published evidence on the topic since 2017 that might inform updates of the definition, diagnosis and management recommendations, as well as produce a digest comprising an update of previous report topics: sex, gender and hormones; epidemiology; pathophysiology; tear film; pain and sensation; iatrogenic causes; and clinical trial methodological recommendations. A particular focus in TFOS DEWS III is to better align dry eye disease management with diagnostic/subclassification features of an individual patient for improved patient care. Collectively, these reports act as a critical reference compendium for clinicians and researcher alike, identifying best (evidence-based) practice and helping guide future research priorities. The goal and expectation is to be able to share TFOS DEWS III with the global community in 2025. |
DREAM Dry Eye Assessment and Management Study. Sparking considerable debate upon publication, this research explored the role of omega-3 fatty acid (OFA) supplements in the treatment of dry eye disease. Many optometrists bristled at its contention that OFAs are no better than placebo, citing their own anecdotal experiences with patients as evidence. Both treatment groups (omega and placebo) improved from baseline, with no statistically significant differences between them.
 |
|
The DREAM study continues to be a source of debate. It found no benefit from omega-3 fatty acid supplements in dry eye, but critics say the placebo itself had a treatment effect, confounding the results. Photo: Jessica Steen, OD. Click image to enlarge. |
Dr. Shovlin acknowledges the controversy behind this study, noting that the placebo it used—olive oil—itself has potential benefits for DED, complicating the results. Despite this, he says, many clinicians continue to endorse the perceived benefits of omega-3 supplements. However, a DREAM extension study revealed no significant worsening of symptoms among patients who stopped omega-3 supplements when compared to those who continued supplement use at 24 months.9
Though there were no statistically significant differences between the active (triglyceride fish oil) and placebo cohorts, there was a 30% improvement in symptoms and some signs, adds Dr. Karpecki, who notes the challenges inherent in “real-world” studies, where it’s necessary to distinguish the effects of a single ingredient from a multitude of other variables.
While the debate surrounding this treatment approach continues, the DREAM study has influenced clinical care. Dr. Sutton suggests that this analysis has called into question the use of oral omega-3 supplements while Dr. Karpecki says it “pointed us to look at other, scientifically proven interventions for dry eye omega-fatty supplements like gamma-linolenic acid (GLA).”
Many clinicians continue to recommend some form of omega supplementation for dry eye, notes Dr. Nichols, “often with the caveat that supplementation may also be useful to manage low-grade systemic inflammation.” Studies like this are very expensive and difficult to do, she points out. Identifying the best placebo and the exact formulation of omega supplementation to use are challenging. “There is a significant placebo effect in many dry eye studies, including this one. I suspect a large study like this—using supplements—will not be done again due to difficulties in study design, identifying appropriate subjects and cost.”
AMD
AREDS Age-Related Eye Disease Study 1 and 2. When a clinical trial’s acronym makes its way onto the labels of consumer products, you know it must be significant. For more than two decades, optometrists have encouraged certain well-selected patients with age-related macular degeneration (AMD) to take dietary supplements in hopes of slowing down the disease’s otherwise inexorable progress.
 |
|
AREDS2 supplements can reduce risk of conversion from dry to wet AMD but the safety of zinc remains controversial. Photo: Carolyn Majcher, OD. Click image to enlarge. |
Exploring the effects of vitamins and minerals on AMD as well as cataract, AREDS and AREDS2 were “the first to show that specific supplements decreased the risk of conversion from intermediate dry AMD to wet AMD,” explains Dr. Sutton. The presumed cataract effect, however, never materialized.
This research, Dr. Gurwood explains, showed that individuals at a “high risk of developing advanced stages of AMD lowered their wet AMD risk by about 25% and their risk of vision loss by about 19% when treated with a high-dose combination of vitamin C, vitamin E, beta-carotene and zinc.”
Sherry Bass, OD, a distinguished teaching professor at SUNY College of Optometry, emphasizes that patients with intermediate AMD are now advised to take AREDS vitamins to reduce risk of progression to choroidal neovascularization and geographic atrophy.
The AREDS studies remain a cornerstone of optometric practice; however, they are not without controversy. For instance, there is ongoing debate regarding the use of genetic testing to determine if a patient should be administered a formulation that contains high amounts of zinc.
Although AREDS2 demonstrated that 25mg of zinc had the same effect on risk reduction as 80mg of zinc, most patients still use the higher dose, Dr. Bass notes, while also highlighting that the American Academy of Ophthalmology (AAO) still does not recommend genetic testing for risk of progression or recommended vitamin (no zinc or zinc). “Since there is controversy and conflicting studies, the AAO needs to revisit this policy.”
Drs. Sutton and Bass agree that additional research is needed to better understand the issues surrounding high zinc formulations and genetic testing, although Dr. Sutton concedes that it would take years to complete.
BDES Beaver Dam Eye Study. Initially funded in 1987 and published in 1992, the BDES aimed to gather data on the prevalence, incidence and potential causes of age-related maculopathy, now known as AMD, states Marc Myers, OD, an optometrist at the Coatesville Veterans Affairs Medical Center in Coatesville, PA.
 |
| The Beaver Dam Eye Study brought increased attention to vigilance for AMD, including careful assessment of drusen. Photo: Jessica Haynes, OD. Click image to enlarge. |
This analysis of over 5,000 subjects from Beaver Dam, MI revealed that “AMD prevalence increases with age and there is strong evidence of a genetic association in this condition,” Dr. Myers explains. An association between cigarette smoking and cataract and AMD was also observed.
Data derived from BDES have changed clinical care. Dr. Myers explains, “Patient examinations evolved to include more thorough medical and family history taking as well as examining the macula for the presence of drusen, geographic atrophy and exudate.”
Discussing the value of these findings today, Dr. Myers says, “When examining adult patients age 65 years and older, the pertinent medical, social and family history findings identified in the BDES remain relevant.” The study continues to inform patient care and research, and although BDES identified patients aged 75 years and up as those with significant risk of the condition, Dr. Myers notes that “we are now aware that the early clinical signs associated with AMD may occur at a much earlier age.”
The BDES has produced more than 360 publications, according to Dr. Myers, and, along with AMD, it has collected extensive epidemiologic information associated with glaucoma, refractive error, retinal vascular disease, vitreoretinal interface abnormalities and retinal emboli.
LUCENTIS TRIALS MARINA, ANCHOR, HORIZON and SEVEN-UP. Collectively, these studies evaluated the efficacy of ranibizumab (Lucentis), the first FDA-approved anti-VEGF drug that actually restored lost vision in patients with wet AMD; a prior anti-VEGF, pegaptanib, at best merely maintained the patient’s visual status. Together, these trials confirmed that Lucentis is a safe and effective therapy for these patients, Dr. Bass says. Based on these findings, ranibizumab was approved by the FDA in 2006 and the landscape of AMD care changed radically.
Patients could either receive monthly injections or follow an off-label “treat and extend” approach, customized to their individual needs, to prevent vision loss, Dr. Bass explains. However, by now most retina specialists have moved on to longer-acting anti-VEGF drugs that allow them to significantly lengthen the treatment interval, reducing the burden on patients and caregivers.
While anti-VEGF drugs dramatically improved the outlook for wet AMD, there are challenges. Patients may still experience vision loss over the long term and the treatment thins the retina, notes Dr. Bass. However, “there are no alternatives at this time to anti-VEGF treatments,” she says, highlighting the need for ongoing study and advancements.
CATT Comparison of Age-Related Macular Degeneration Treatments Trials. Rampant off-label use of bevacizumab, an anti-VEGF agent structurally similar to ranibizumab but substantially cheaper, upended Lucentis’s market position in the early days of the anti-VEGF era. To settle the matter, this NEI-funded study compared the two drugs head to head.
A multicenter, randomized clinical trial of more than 1,200 patients with wet AMD, CATT found that “bevacizumab was non-inferior to ranibizumab” for the treatment of this condition, according to Dr. Bass. This is a significant finding given that bevacizumab is less expensive than ranibizumab, offering a more affordable treatment option to patients.
The presentation of CATT’s findings to a packed house at the ARVO conference in May 2011 attracted the attention of consumer media and validated to many that the far cheaper Avastin was a perfectly acceptable alternative to Lucentis. The Department of Veterans Affairs even used CATT as justification for Avastin to be used at its network of VA centers, a tacit endorsement by a federal agency of a contentious off-label use.
In light of the cost implications, these findings had a significant impact on clinical practice. Dr. Bass explains that patients whose insurance would not cover the cost of ranibizumab now have a more affordable option that may be accessible through their insurance. “Therefore, these patients had a chance of having their wet AMD treated even if they were not eligible or could not afford ranibizumab.”
Many practices continue to use bevacizumab today, and numerous patients have delayed vision loss from wet AMD, Dr. Bass reiterates while discussing the ongoing value of these studies. Future research must continue to develop cheaper and more effective treatments for patients with this condition, she emphasizes.
VIEW VIEW 1 and VIEW 2 Trials. With the anti-VEGF era in full swing by the early 2010s, these trials investigated another agent, aflibercept (Eylea), to treat wet AMD. Aflibercept functions by binding to VEGF-A and VEGF-B, preventing the growth of abnormal blood vessels in the eye, a key feature of wet AMD.
These studies, which were conducted as two parallel, randomized, double-masked Phase III trials, enrolled more than 2,400 patients. VIEW 1 and 2 demonstrated that aflibercept administered every eight weeks, after three initial monthly doses, was as effective as ranibizumab in maintaining vision among this patient population. Suddenly, AMD patients only needed to receive an injection about half as often as before.
“Patients whose wet AMD may not have been well controlled with other anti-VEGF agents like ranibizumab and bevacizumab are better controlled with Eylea and Eylea-HD,” Dr. Bass says, while acknowledging that the cost of the drug is a challenge and not all insurance plans will cover its use. “We need to develop an anti-VEGF that is as effective as aflibercept but cheaper and more accessible.”
Diabetic Eye Disease
ETDRS Early Treatment Diabetic Retinopathy Study. It’s rare for a clinical trial published back when George H.W. Bush was president to remain relevant, but one could make a strong case for that with 1991’s ETDRS. Setting aside its clinical implications in disease care, the study “produced the ETDRS best-corrected visual acuity (BCVA) method,” which is used in almost all FDA clinical trials, remarks Dr. Pucker. But the changes to diabetic eye care it ushered in remain vital today as well.
ETDRS was designed to address key questions around the management of nonproliferative or early proliferative diabetic retinopathy (PDR) while exploring the effectiveness of early panretinal photocoagulation (PRP) and aspirin therapy for vision loss prevention in DR patients.
Before ETDRS, half of patients with PDR were at risk of blindness within five years. ETDRS led to a significant improvement in outcomes, with progression to legal blindness dropping to less than 5% over five years and severe vision loss reduced to 1% in the study population, according to Dr. Gurwood.1 In fact, due to the overwhelmingly positive results of the study, which support the use of PRP for PDR, the analysis was stopped early. Dr. Bass notes, “This has reduced the blindness that has plagued patients for years who had PDR.”
Dr. Myers adds that scatter photocoagulation led to a reduction in severe vision loss among patients with severe nonproliferative or early proliferative retinopathy, while focal photocoagulation helped decrease moderate vision loss in diabetic macular edema (DME). However, aspirin did not prove effective in reducing vision loss or slowing the progression of retinopathy. Despite this, it did not raise the risk of retinopathy, so patients can continue using it without concern.
While acknowledging the shift from grid/focal laser therapy to anti-VEGF injections for DME, Dr. Bass asserts that the impact of ETDRS continues today. “This treatment is still being used for PDR, and often patients who may not come back for anti-VEGF injections would get a ‘big bang for the buck’ with even one laser treatment,” she observes. Dr. Myers adds that ETDRS “clarified the timing for when laser photocoagulation should be performed,” underscoring its role in defining treatment guidelines.
Research continues to build on the groundwork set up by ETDRS. This includes, according to Dr. Myers, ongoing studies on subthreshold micropulse laser combined with anti-VEGF injections for DME. The goal is to minimize retinal damage and reduce the number of injections, possibly improving outcomes.
PROTOCOL I DRCR Protocol I. This research effort, spearheaded by the Diabetic Retinopathy Clinical Research (DRCR) Retina Network, compared the efficacy of laser treatment and anti-VEGF therapy, specifically ranibizumab, for the management of DME, and its influence on optometric practice can still be seen today.
“This study found that the proportion of eyes with significant vision improvement was greater in the ranibizumab group who deferred laser than the group that had ranibizumab and prompt laser,” Dr. Bass outlines. This outcome highlighted the effectiveness of anti-VEGF therapy alone, moving the field away from laser in these cases. Given that PRP “only destroys tissue,” this was an important development, according to Dr. Bass.
Protocol I’s conclusions hold true today. The preference for anti-VEGF therapy over laser is now standard practice, demonstrating the study’s enduring value and influence. Further evolution, such as research focused on developing more affordable anti-VEGF agents to treat DME, is still needed, Dr. Bass suggests.
 |
| Although PRP is far less often used in the anti-VEGF era, the ETDRS findings of its value were transformative at the time. Photo: Christine Sindt, OD. Click image to enlarge. |
PROTOCOL V DRCR Protocol V. While anti-VEGF therapy is an effective treatment for a variety of ocular conditions, it can be time-consuming, costly and risky. “DRCR Protocol V provides evidence that a conservative, watchful waiting approach can be safely used for a large portion of DME patients,” notes Sara Weidmayer, OD, who practices at the LTC Charles S. Kettles VA Medical Center in Ann Arbor, MI.
This analysis compared the efficacy and safety of three management strategies for patients with center-involved (CI) DME and good visual acuity: aflibercept injections, laser photocoagulation and observation. If vision worsened in the laser or observation groups, aflibercept was used.
Data showed that the mean visual acuity was 20/20 across all groups at two years, with only minor differences in vision loss, reports Dr. Weidmayer. “Because of this data, we understand that it is safe to just monitor CI-DME unless it is—or becomes—visually significant.”
DRCR Protocol V’s findings are still integral to optometric decision-making. In her own practice, Dr. Weidmayer no longer refers most of her center-involved, not visually significant (CI-NVS) diabetic macular edema patients to a retina specialist, but rather employs the watchful waiting approach.
There are a number of future avenues that could build on this work. For instance, Drs. Bass and Weidmayer agree that following patients for more than two years would be valuable. Additionally, Dr. Weidmayer suggests stratifying outcomes based on the amount of CI-DME and/or DR stage, as well as establishing clearer guidelines on follow-up intervals for monitoring CI-NVS DME.
ANTI-VEGF FOR DME RIDE, RISE, VIVID and VISTA Trials. These studies, which explored the use of ranibizumab and aflibercept in DME management, offered further insight into the long-term impact of anti-VEGF in this patient population.
The Phase III randomized multicenter RIDE and RISE trials demonstrated that the significant visual acuity gains and improvements in retinal anatomy achieved with ranibizumab at 24 months were maintained through three years. Ocular and systemic safety outcomes remained generally consistent with observations made at month 24.10
Similarly, the Phase III VIVID and VISTA trials confirmed that aflibercept is highly effective in improving vision in DME patients over a similar time period, offering an alternative to laser therapy.11
Data from these studies showed clinicians the sustained efficacy that ranibizumab and aflibercept offer DME patients a reliable treatment option associated with long-term positive outcomes.
Dr. Bass poses an important question for future study: “Could these treatment effects last even longer than 36 months as long as the diabetes remains under good control?” Exploring this avenue of research could lead to further improvements in management of diabetic macular edema, potentially prolonging the benefits of treatment and improving patient outcomes.
Uveitis
MUST Multicenter Uveitis Steroid Treatment Trial. Posterior uveitis can be a minefield, full of intractable visual and ocular health consequences with few good treatment options. The 2011 MUST trial demonstrated just how tough it can be to develop an acceptable regimen. This prospective randomized controlled trial spanned 23 tertiary care centers across three continents and explored the efficacy of local vs. systemic immunosuppression.
Data showed that local immunosuppression with intravitreal fluocinolone acetonide (FA) was equally effective to systemic immunosuppression in controlling intraocular inflammation, resulting in statistically similar outcomes in visual acuity, cystoid macular edema and uveitis control. Unfortunately, the adverse effects were substantial and, ultimately, a deal-breaker in most cases.
“Intravitreal FA had higher rates of ocular morbidity with increased rate of cataract formation, change in IOP by >10mm Hg and incidence of glaucomatous damage requiring medical or surgical intervention,” notes Rami Aboumourad, OD, of Bascom Palmer Eye Institute in Miami. “Theoretically, steroid responsiveness could be mitigated with a shorter duration steroid such as intravitreal triamcinolone before proceeding to the FA implant, but this is not foolproof and also does not address the cataract risk.”
While the FA implant provided rapid inflammation control, it was only cost-effective when used unilaterally, and it posed significant ophthalmic side effects, including a high risk of cataract within 24 months, according to Dr. Aboumourad.
“Intravitreal FA showed only marginally better quality-of-life scores compared with systemic treatment, and there were similar rates of systemic adverse events,” he says. “Ultimately, the study was unable to demonstrate superiority of intravitreal FA as compared with systemic therapy.”
Why care about the MUST trial? Dr. Aboumourad stresses the necessity of ongoing IOP monitoring for patients receiving intravitreal FA, especially those with implants rather than intravitreal drug delivery. This research demonstrated the effectiveness of intravitreal corticosteroids for the management of intraocular inflammation while also shedding light on the associated risks and limitations.
A Phase IV trial is currently evaluating a lower-dose FA implant (0.18mg rather than 0.59mg). “We look forward to seeing if the adjusted dose yields effective inflammatory control with improved side effect profile,” notes Dr. Aboumourad. Other trials are exploring steroid-sparing drugs delivered intravitreally.
 |
|
The SUN consensus established definitions and criteria for birdshot chorioretinitis and dozens of other clinical entities. Photo: Rami Aboumourad, OD. Click image to enlarge. |
SUN Standardization of Uveitis Nomenclature. This unique initiative wasn’t a study per se but rather a meeting of the minds aimed at dispelling the ambiguity that had previously surrounded conversations about uveitis. Dr. Rixon notes, “Prior to SUN, there was a lack of standardization in terminology used to classify the uveitides, which led to limited agreement amongst providers.”
A collaborative effort among global experts, SUN—published in 2005 and updated since—created a consensus on terminology, inflammation grading and classification criteria, offering clarity on how to approach uveitis care. The effort “provided the opportunity for practitioners to have greater consistency in diagnosis and management of patients with uveitis, especially when grading uveitic activity,” Dr. Rixon remarks.
To this day, SUN arguably remains the gold standard in uveitis classification, according to Dr. Rixon. The criteria established by the SUN group are widely recognized and continue to inform both clinical practice and research in the management of uveitis.
What comes next? Dr. Rixon suggests future research could help classify specific uveitis entities, and mentions that the SUN working group developed classification criteria for 25 of the most common forms, using characteristics from a minimum of 250 cases/disease.12 The SUN initiative offers a comprehensive framework for the classification and management of uveitis while paving the way for future research and clinical progress.
SITE Systemic Immunosuppressive Therapy for Eye Diseases. This investigation delved into the long-term outcomes and safety of systemic immunosuppressive therapy for patients with non-infectious uveitis. “The largest prospective review within uveitis,” states Dr. Aboumourad, SITE included 9,250 patients from five tertiary care centers in the US, with an amazing 30 years of follow-up data in some cases.
The goal was to determine whether the use of immunosuppressives affected mortality and/or fatal malignancy risk among uveitis patients, comparing various treatments, including oral corticosteroids, antimetabolites, calcineurin inhibitors and alkylating agents.
Data revealed that patients treated with oral corticosteroids, antimetabolites (azathioprine, methotrexate, mycophenolate mofetil) and calcineurin inhibitors (cyclosporine) all had similar overall and cancer-related mortality rates compared with those not exposed to immunosuppression, Dr. Aboumourad summarizes. “Control of ocular inflammation was sustained in 62.2%, 66.0%, 73.1%, 51.9% and 76.3% of patients on azathioprine, methotrexate, mycophenolate mofetil, cyclosporine and cyclophosphamide, respectively,” he explains. “All drugs showed similar loss of inflammatory control with the cessation of prednisone.”
Alkylating agents (cyclophosphamide) showed a borderline increase in cancer-related mortality but this was not statistically significant, he adds. Anti-TNF-α biologic agents were associated with a significantly increased rate of overall and cancer-related mortality, making them an undesirable first-line option unless there is a severe degree of inflammation.
Given the study’s insights into the long-term safety profile of corticosteroids, antimetabolites and calcineurin inhibitors, this research remains an important component of clinical practice, argues Dr. Aboumourad.
“Of note, cyclosporine was the least effective at controlling inflammation and has the least favorable side effect profile of the aforementioned drugs and is therefore not a preferred drug for the management of uveitis.” This particular study cohort underwent subsequent subanalysis that showed the following:
- Immunosuppression improved uveitic macular edema and final visual acuity.
- Behçet-associated uveitis patients with uncontrolled intraocular inflammation, posterior synechiae, hypotony or elevated IOP were all associated with increased risk of vision loss.
- Juvenile idiopathic arthritis–associated uveitis with uncontrolled inflammation resulted in vision loss but could be mitigated with immunosuppression.
- Anti-TNF-α agents were effective at controlling inflammation in 75% of pediatric uveitis patients.
- Hypopyon is relatively rare in uveitis and is associated with Behçet disease, HLA-B27 positivity and spondyloarthropathies. The presence of uveitis-associated hypopyon was not associated with increased risk of structural complications or vision loss.
- Relative risk of steroid-induced hyperglycemia was 4.4-times higher than patients not taking corticosteroids. Cumulative risk was 1% per year.
While discussing future areas of interest, Dr. Aboumourad highlights the ongoing challenges associated with achieving optimal inflammation control while managing both systemic and ophthalmic side effects. “Further research is needed to better understand the long-term safety of anti-TNF-α biologic agents (e.g., adalimumab) and as such there are multiple ongoing Phase IV trials studying its use in a variety of uveitic entities. Continued research will allow us to better identify a tailored algorithmic approach to each patient’s needs to control their specific type of intraocular inflammation.”
 |
|
The SITE trial’s emphasis on long-term anti-inflammatory use helped establish protocols for difficult cases like this sympathetic ophthalmia, which was managed with topical prednisolone, sub-Tenon’s Kenalog, intravenous infliximab, oral prednisone and oral mycophenolate. Photo: Rami Aboumourad, OD. Click image to enlarge. |
Retinal Vein Occlusion
STEROIDS FOR RVO SCORE and GENEVA. Both of these studies enhanced our understanding of treatment options for macular edema secondary to central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO). The trials focused on the efficacy of intravitreal triamcinolone (SCORE) and dexamethasone (GENEVA). Discussing the findings from the SCORE study, Dr. Bass notes, “There was no difference in visual acuity at 12 months between the group receiving grid photocoagulation vs. 1mg and 4mg triamcinolone; however, there were more adverse reactions with the 4mg triamcinolone group.”
 |
| Macular edema due to CRVO was managed with intravitreal steroids in the SCORE and GENEVA trials. Photo: Jessica Haynes, OD. Click image to enlarge. |
In GENEVA, which evaluated a dexamethasone implant (0.7mg), “the improvement in VA did not last beyond 60 days,” notes Dr. Bass, who suggests that while this approach provided short-term benefits, its effects were not sustained over the long term. SCORE and GENEVA shed light on potential alternative treatments for patients who do not respond to anti-VEGF for macular edema secondary to CRVO and BRVO, she suggests.
Remarking on their relevance, Dr. Sutton notes, “SCORE examined use of steroids before anti-VEGF was prominent,” while Dr. Bass adds, “Clinically, given today’s better anti-VEGF agents and the side effects of steroids, more practices are injecting anti-VEGF to treat macular edema associated with RVO.”
Future research could investigate the potential advantages of steroid injections over anti-VEGF, possibly focusing on cost-effectiveness, Dr. Bass says; however, studies would have to demonstrate the benefits of this approach to reconsider the role of steroids in treating macular edema.
ANTI-VEGF FOR RVO BRAVO, CRUISE, COPERNICUS, GALILEO. This group of studies helped shape current clinical practices around the treatment of macular edema secondary to RVO. They assessed the efficacy and safety of anti-VEGF therapies in this patient population.
Ranibizumab is an effective treatment option for macular edema secondary to BRVO, based on findings from BRAVO and CRUISE, demonstrating that “patients treated with ranibizumab at 12 months gained more visual acuity and had better reading speed,” Dr. Bass outlines. “Patients needed treatments more than every three months since VA would decline at three months.”
The COPERNICUS and GALILEO trials focused on aflibercept, examining the connection between central subfield macular thickness and visual acuity. The correlation was weak, which indicates, according to Dr. Bass, “that central subfield macular thickness should not be used to determine the success of treatment or potential VA.”
While these studies get into the weeds of retina subspecialty care, they do have implications for the optometrist and their practice in referral protocols and patient education. “Ranibizumab injections more frequently than just one in three months is effective in improving VA in patients with macular edema in RVO,” advises Dr. Bass. Additionally, she emphasizes, “while central macular thickness is measured after treatment with aflibercept, it is not a substitute for determining success and VA should be used.”
When asked if this data remain relevant, Dr. Bass affirms their value, while also noting that although central macular thickness is followed after treatment, “clinicians rely on BCVA.” This ensures treatment decisions are informed by the most precise evaluation of visual function.
Collectively, the BRAVO, CRUISE, COPERNICUS and GALILEO studies offered additional insights into the management of macular edema secondary to retinal vein occlusions, highlighting the value of consistent treatment and thorough visual assessments.
Inherited Retinal Disease
LUXTURNA Luxturna Trials. “A huge win—with a huge price tag” was everyone’s hot take in 2017 when the FDA approved voretigene neparvovec-rzyl (Luxturna) to treat RPE65 mutation–associated retinal dystrophy in Leber’s congenital amaurosis (LCA) and retinitis pigmentosa (RP). The world’s first approved gene therapy, Luxturna costs $850,000 for a course of treatment.
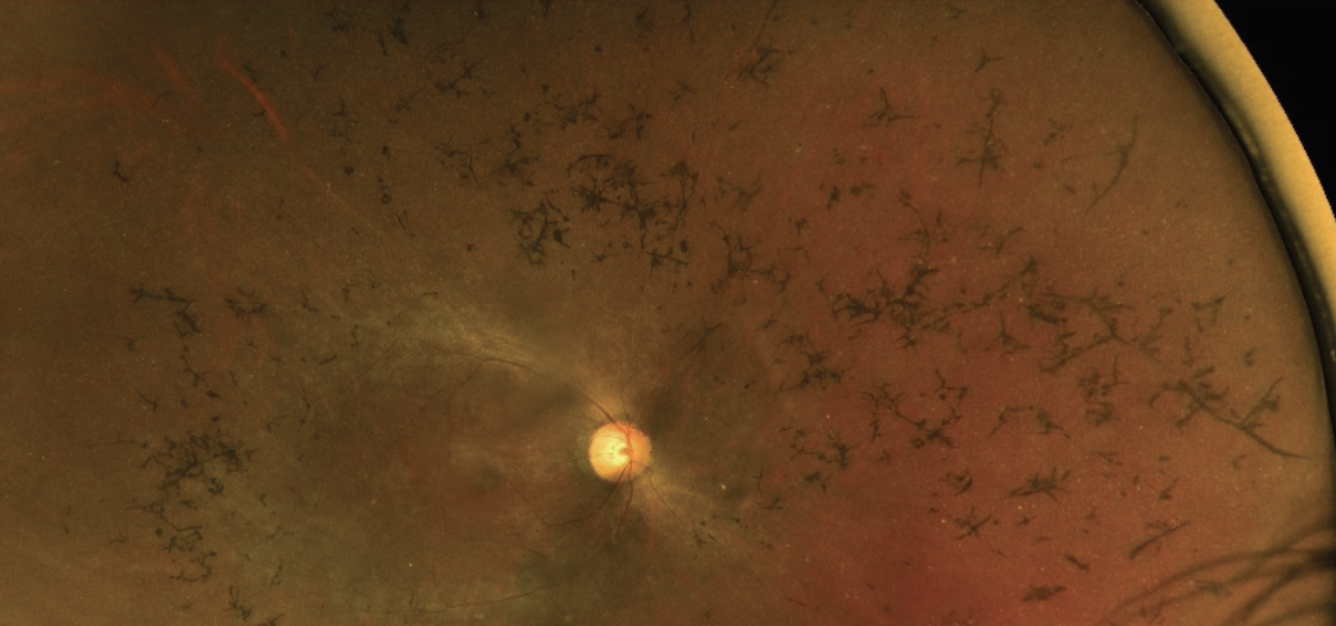 |
| Retinitis pigmentosa patients with the RPE65 mutation are potential candidates for Luxturna gene therapy. Photo: Mohammad Rafieetary, OD. Click image to enlarge. |
Sharing the key takeaways, Dr. Sutton says, “These trials showed that Luxturna injection can improve visual function in patients who are homozygous for RPE65 mutation in RP or LCA.” The studies showed that transporting a normal copy of the RPE65 gene subretinally using a viral vector to carry the gene could have an effect on the patient’s ability to navigate in dim environments, Dr. Bass suggests, adding that this is currently the only FDA-approved therapy for LCA.
These trials had a tremendous impact. “They started a treatment paradigm for these patients,” says Dr. Sutton. Elaborating further, Dr. Bass adds, “Many patients were able to see better in dim illumination after the treatment with RPE65 gene therapy. The gene continues to express itself for many years, even though the mutation that caused the retinal destruction is still present and hence the disease is still progressing.”
Both Drs. Sutton and Bass agree that the Luxturna FDA trials still play an important part in clinical care. Although only applicable to about 300 patients in the US with LCA and biallelic RPE65 mutations, this treatment is revolutionary and one of a kind, according to Dr. Bass.
As the field of gene therapy continues, so does the potential impact for patients with inherited retinal disease. There are a number of other gene therapy trials underway, especially for different types of LCA. “These therapies range from surgical insertion of the virus vector under the retina to intravitreal injections of oligonucleotides that replace defective mRNA, but do not correct the defective DNA to CRISPR-case 9 gene editing technologies,” outlines Dr. Bass. Most of these studies are in early phases, focusing on safety and tolerability.
GEMINI GEMINI Study. Could choroideremia be the next big success story in gene therapy for inherited retinal disease? This progressive, untreatable retinal degeneration predominantly affects young men and ultimately results in vision loss. The Phase II GEMINI study was initiated to explore bilateral sequential administration of timrepigene emparvovec, a gene therapy, in adult males with genetically confirmed choroideremia.
“Visual acuity was generally maintained in both eyes, independent of intrasurgery window duration, even after bilateral retinal detachment and subretinal injection,” the study authors reported this August in a paper on the ongoing research.13 “Bilateral treatment was well tolerated, with predominantly mild or moderate treatment-emergent adverse events and a low rate of serious surgical complications (7.6%),” the paper states.
The gene therapy timrepigene emparvovec was subsequently evaluated in the Phase III STAR trial; however, “it did not meet the primary endpoint of improvement in VA of greater than 15 ETDRS letters,” according to Dr. Bass.
The GEMINI study, as well as the STAR trial, highlights the challenges that come with developing effective gene therapies. Although further study is needed, this research offers important insights into the safety and limitations of gene therapy approaches.
Neuro-ophthalmic Disease
ONTT Optic Neuritis Treatment Trial. What’s the role of steroid therapy in optic neuritis, and how might it affect the development of multiple sclerosis (MS)? That was the ONTT’s mandate. The trial compared three treatment groups: oral prednisone, intravenous methylprednisolone followed by oral prednisone, and an oral placebo.
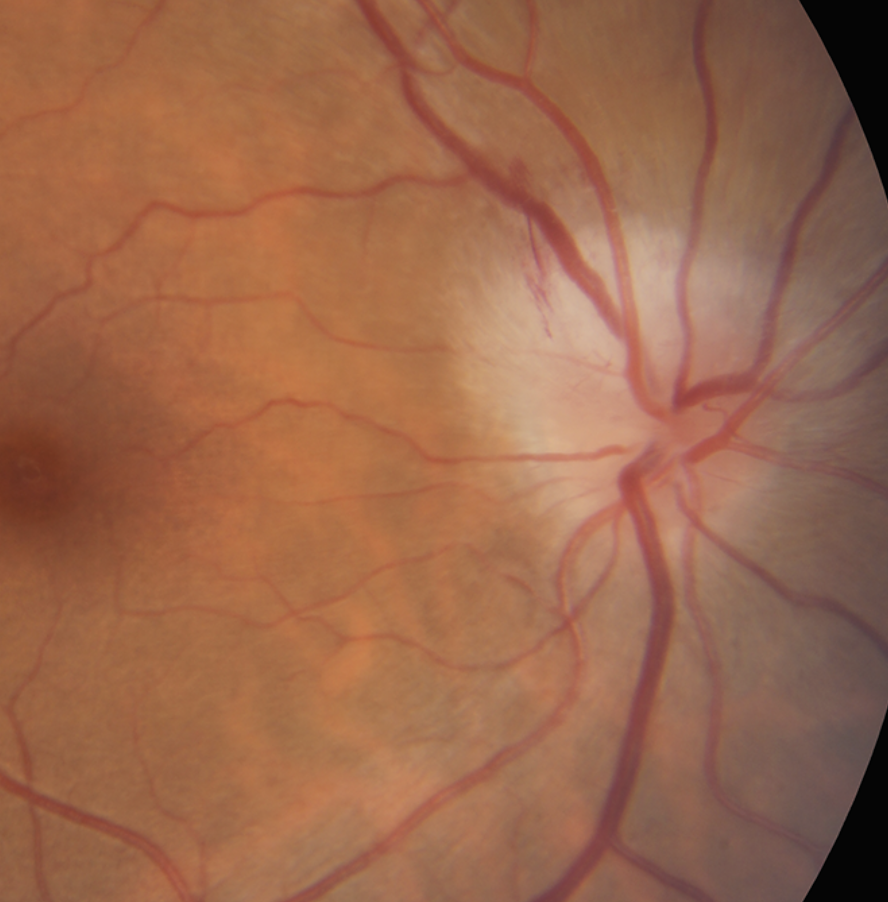 |
|
The ONTT explored the safety and efficacy of steroid therapy to treat optic neuritis in patients with multiple sclerosis. Photo: Nate Lighthizer, OD. Click image to enlarge. |
According to Dr. Weidmayer, before the trial, oral steroids had been the routine treatment for acute optic neuritis. However, the ONTT’s findings led to significant changes in clinical practice. The study found that patients treated with oral prednisone alone experienced twice the rate of optic neuritis recurrence compared to the other groups, leading to the swift abandonment of oral prednisone as a standard treatment. Dr. Sutton highlights that this trial established a regimen of IV steroids followed by oral steroids as superior to oral steroids alone.
The ONTT further demonstrated that IV methylprednisolone reduced the short-term risk of developing MS, especially in patients with MRI abnormalities suggestive of demyelination. However, this lower risk was not sustained beyond two years. As Dr. Weidmayer points out, we now know that high-risk MRI findings at the time of optic neuritis presentation are the best predictors of future MS development.
Although the ONTT was conducted in the early 1990s, its findings remain relevant today. Dr. Sutton notes that high-dose oral steroids are sometimes used in place of IV steroids, reflecting evolving treatment practices.
Kelly Malloy, OD, a professor and the director of the neuro-ophthalmic disease service at Pennsylvania College of Optometry at Salus University in Philadelphia, adds that the long-term follow-up data from the ONTT, spanning 15 years, helps guide patient education. Specifically, patients presenting with isolated optic neuritis who show white matter changes on their initial MRI have a 70% chance of developing clinically definite MS within 15 years, while those without such changes face only a 25% risk.
Looking ahead, Dr. Sutton suggests that repeating the trial with high-dose oral steroids vs. IV steroids could provide further insights into the optimal treatment for optic neuritis. Ongoing research may continue to refine treatment strategies, particularly as new therapies and diagnostic tools emerge.
IIHTT IIH Treatment Trial. This study sought to either confirm or dispel the presumed validity of prescribing acetazolamide (Diamox) to manage idiopathic intracranial hypertension (IIH), particularly in patients with mild visual loss, as had been commonly done prior to the study. In short, Diamox prevailed.
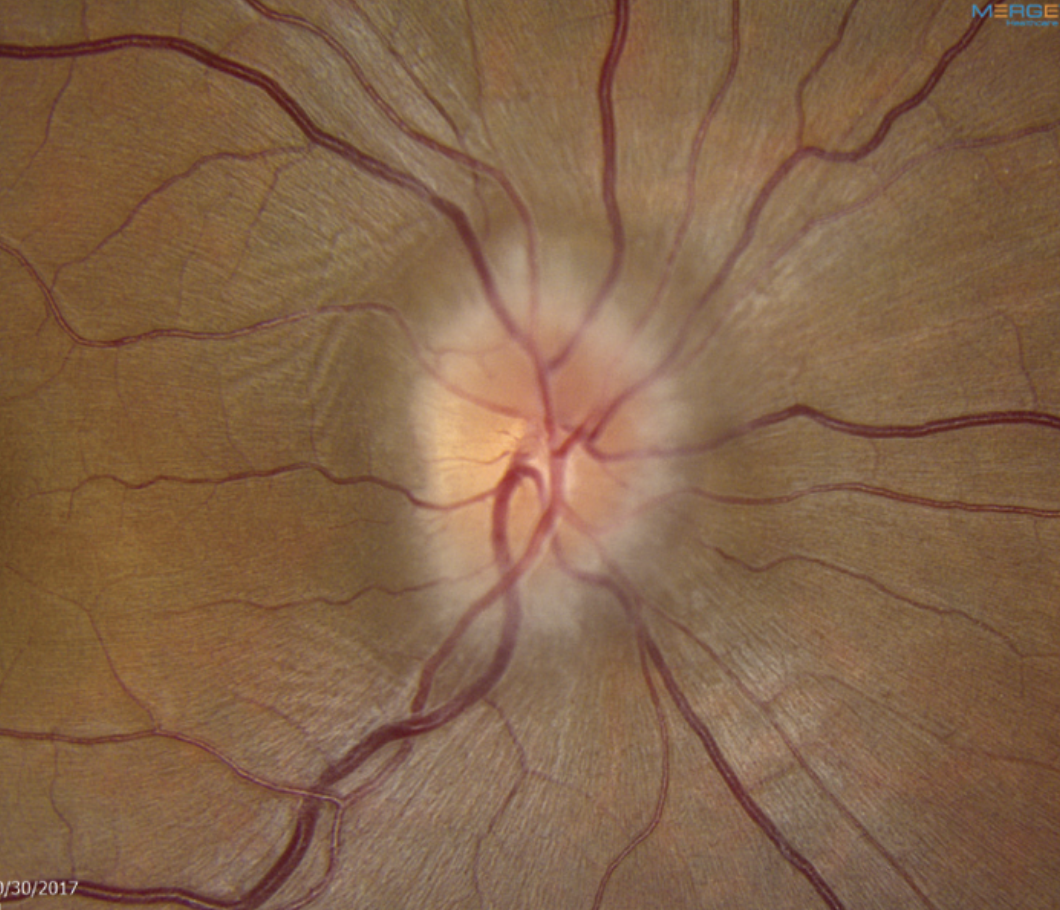 |
|
The IIH Treatment Trial validated the use of Diamox in affected patients. It also highlighted the value of weight loss efforts. Photo: Mark Dunbar, OD. Click image to enlarge. |
The IIHTT included two arms, one with acetazolamide and the other with placebo, both combined with weight reduction and a low-sodium diet. Data showed that the acetazolamide-plus-diet group experienced a statistically significant improvement in visual field mean deviation, with most changes occurring within the first month, Dr. Malloy explains. There was also a greater decrease in OCT optic disc volume in the treatment group compared to the placebo group. However, there was no significant difference in visual acuity improvement between the two.
Dr. Malloy notes, “It gave us proof that Diamox usage in concert with weight loss was the best approach and gave us justification to recommend this treatment to our patients.”
The acetazolamide group showed improvement not only in visual fields but also in papilledema grade, quality of life measures and cerebrospinal fluid (CSF) pressure. In addition, those in the acetazolamide arm actually lost more weight (6% of total body) than those in the placebo arm (3.2%.) “So, from this we can gather that acetazolamide helps promote weight loss,” Dr. Malloy says. The long-term (four-year) follow-up was also important in proving that “there were no significant associated metabolic or renal events with long-term acetazolamide use, and therefore, repeat laboratory testing was not indicated in these patients.”
IIHTT’s results are still relevant today, says Dr. Malloy—“in fact, it becomes more relevant as the population becomes more overweight and obese as a whole and we see more patients with IIH.” This study helps ODs educate patients on the need for a combination of weight loss and acetazolamide if they start to have visual field loss secondary to IIH. “However,” Dr. Malloy elaborates, “prior to any documented field loss, we may be able to recommend weight loss alone. If successful, the goal would be that those patients may not need acetazolamide at all.”
Moving forward, research could expand on IIHTT, Dr. Malloy suggests, by exploring best practices for patients with moderate and severe vision loss. Another helpful investigation would be a comparison between medical management and surgical options, such as optic nerve sheath fenestration and CSF shunting.
OPTIC OPTIC Trial. This randomized, placebo-controlled trial galvanized management of thyroid eye disease (TED) by ushering in approval of the first purpose-built medication to treat it—teprotumumab (Tepezza). “This study changed the thinking about TED and showed that biologics do have a role in TED treatment and management,” Dr. Malloy shares. Prior to this study, treatment for TED was either steroids, radiation or surgery.
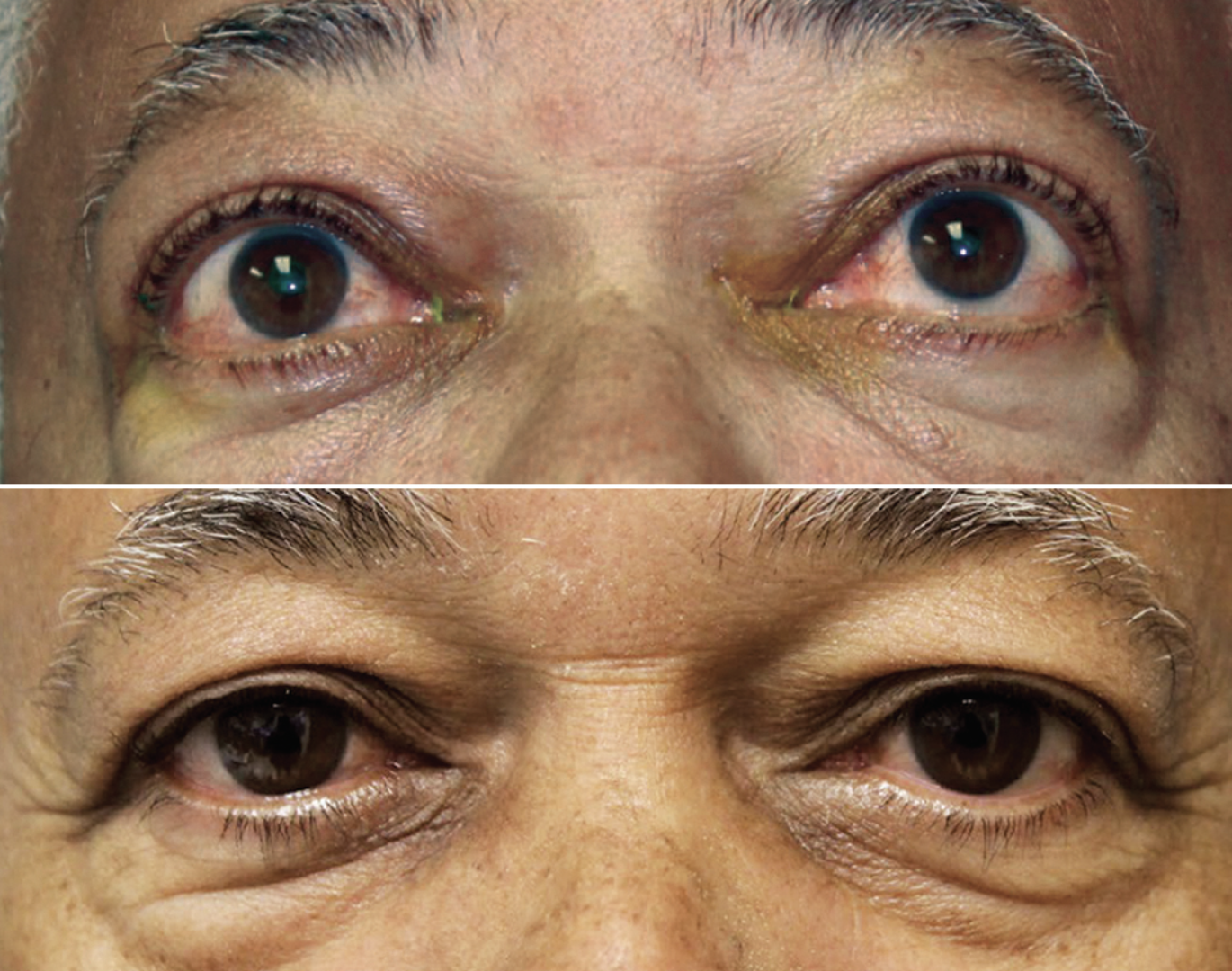 |
|
The OPTIC trial demonstrated Tepezza’s efficacy in improving signs of proptosis in TED patients. Photo: Jacob Lang, OD. Click image to enlarge. |
Researchers evaluated the efficacy and safety of teprotumumab, an insulin-like growth factor-1 receptor inhibitor, compared to a placebo among patients with moderate-to-severe clinically active TED. Patients were randomly assigned to receive either teprotumumab or a placebo, with the treatment group showing a significant reduction in proptosis.
“The majority of patients receiving the teprotumumab had a 2mm reduction in proptosis,” notes Dr. Malloy. Among patients in the treated group, 83% met the primary outcome compared to just 10% in the placebo cohort. Tepezza blocks secretion of inflammatory cytokines, thereby mediating reduction in extraocular muscle volume as well as the effects on orbital fat, both of which contribute to a reduction in proptosis and also diplopia.
The trial also identified common adverse events, such as muscle spasm, hyperglycemia, hearing impairment and exacerbation of pre-existing inflammatory bowel disease.
While the results are still relevant, Dr. Malloy notes that “this initial study looked only at patients with clinically active TED. Additional subsequent studies have since shown that teprotumumab also has a role in the treatment and management of chronic TED as well.”
Although the study was a “game changer,” Dr. Malloy emphasizes that the first line of treatment for TED still involves managing underlying thyroid dysfunction, with surgical options remaining viable depending on severity.
Future research could explore longer-term follow-up data to determine the safety and efficacy of multiple rounds of treatment. Dr. Malloy suggests investigating alternative administration routes, such as oral medication, and studying other components of the TED pathophysiological process to identify additional or complementary treatment options.
Myopia Control
ATOM Atropine for the Treatment of Myopia 1, 2 and 3 Trials. Although use of atropine for controlling myopia dates back to the mid-1800s, the modern era of myopia management began with the publication of the Atropine for the Treatment of Myopia (ATOM) study in 2006. A follow-up (ATOM2) arrived in 2015 and the latest—ATOM3—is underway now. Together, this body of research explores the impact of atropine in various concentrations.
 |
|
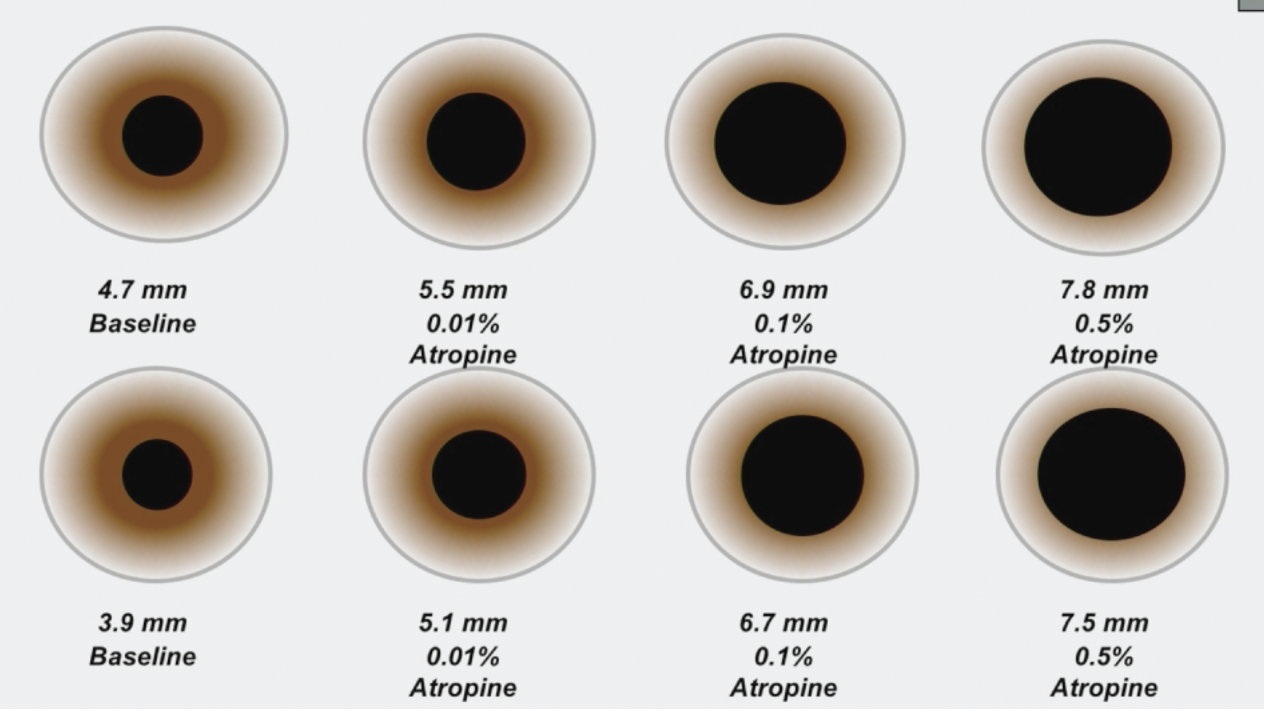
Many studies show atropine’s value in mitigating myopia progression, but optimal dosing to avoid adverse effects remains a point of contention. The ATOM2 study tested three concentrations: 0.01%, 0.1% and 0.5%. Photo: Donald Tan, MD. Click image to enlarge. |
ATOM1 tested 1% atropine in children aged six to 12. While it suggested this approach can reduce myopia progression, side effects of glare, photophobia and blurred near vision prompted investigation of lower concentrations in the subsequent ATOM2 study, which evaluated 0.5%, 0.1% and 0.01% versions. At two years, axial elongation and myopia progression were greater with lower concentrations (0.27mm, 0.30D with 0.5%; 0.28mm, 0.38D with 0.1% ; and 0.41mm, 0.49D with 0.01%).
This clear dose-response must be balanced against side effects and tolerability levels appropriate for children. Among a range of low-dose options studied in the ATOM trials and others, research results and anecdotal practitioner preferences have varied. Nevertheless, these efforts demonstrated the effectiveness of 0.01% atropine in slowing disease progression with fewer side effects. Leading to a shift in clinical practice, “these trials spurred the use of low-dose atropine (0.01%) for myopia management,” says Dr. Pucker.
Unlike the prior studies, ATOM3 focused on whether atropine could be used as a preventive treatment for children at risk of developing myopia. While we are still waiting for data to be released, Erin Tomiyama, OD, PhD, an assistant professor of optometry at Marshall B. Ketchum University, believes that results “will be interesting whether or not they support the use of atropine in pre-myopia.”
The ATOM trials were formative to today’s myopia management landscape, making low-dose atropine one of the most commonly used options, according to Dr. Pucker. Researchers continue to investigate optimal formulations while also trying to determine which patients will benefit from treatment, notes Dr. Pucker.
LAMP Low-Concentration Atropine for Myopia Progression Study. Another research effort examining different concentrations of atropine, LAMP suggested 0.05% is the optimal dosage. This study compared 0.05%, 0.025% and 0.01% concentrations of atropine to determine the most effective dosage with an acceptable safety profile. “Results from this study led to shift away from 0.01% atropine to 0.02% or 0.05% atropine,” says Dr. Pucker.
Despite ongoing questions about the long-term efficacy of low-dose atropine, the LAMP study is “one of the first and few to compare these dosages over a three-year period,” Dr. Tomiyama adds. Ongoing research is critical, including more information on the best formulations, advises Dr. Pucker. “One issue in this area has been the need for using compounding pharmacies. This will be negated when an FDA-approved product is released.”
In Dr. Tomiyama’s opinion, future analyses should take a closer look at the long-term effects of atropine. “The ATLAS study—a long-term follow-up of ATOM1 and ATOM2—showed no long-term effects, which is discouraging for patients and providers to continue to prescribe atropine.”
LAMP indicated that “we can delay the need for glasses in children who are likely to become myopic by giving them daily drops of low-concentration atropine,” says Jeff Walline, OD, PhD, acting dean and a distinguished professor at Ohio State University College of Optometry. He points out that LAMP suggests “0.05%, but not 0.01%, atropine can delay the onset of myopia in children.”
While these results hold promise, widespread adoption of 0.05% atropine for pre-myopes has been limited. Aaron Salzano, OD, an assistant professor at Pacific University College of Optometry, notes, “More evidence is needed on truly long-term efficacy, such as the ATLAS study, and safety data with 10+ years of atropine use is warranted before some practitioners will feel comfortable prescribing atropine broadly.”
The LAMP study has spurred further research into interventions for pre-myopes, Dr. Salzano says, while highlighting the importance of determining which modalities best prevent progression while minimizing side effects.
CLEERE Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error. “This study showed that we can predict with reasonable accuracy who will become myopic based on measurements of refractive error alone,” notes Dr. Walline. This enables optometrists to offer targeted interventions to children at risk of developing myopia, potentially delaying its onset.
CLEERE sought to understand the risk factors for onset and progression of myopia in children from diverse ethnic backgrounds and provided further insight into epidemiology. This was also the first high-quality study to describe protective effects of time outdoors, according to Dr. Pucker, supporting increased emphasis on outdoor activities as a preventive measure.
CLEERE also led to the understanding that “at a young age (six to 11), children should still be hyperopic with an adequate hyperopic buffer to prevent them from developing myopia,” adds Dr. Tomiyama.
Elaborating on the continued relevance of this research, Dr. Salzano notes, “CLEERE established the basis for pre-myopia, which is one of the major areas of study in preventing myopic progression.” He explains that when the International Myopia Institute defined pre-myopia, it primarily cited the CLEERE study as the basis for this definition. “CLEERE does not use the term ‘pre-myopia,’ but rather said that the best predictor of future myopia is refractive error at a given age; namely, spherical equivalent of ≤+0.75D at age six, ≤+0.50D at age seven to eight, ≤+0.25D at age nine to 10 and ≤+0.00D at 11 years of age.”
In fact, many studies are currently building on the CLEERE study’s foundation. “Since we know these risk factors, how can they be modified to prevent myopic development? This is the basis of numerous studies trying to treat pre-myopes,” says Dr. Salzano.
BLINK Bifocal Lenses In Nearsighted Kids. This study yielded a greater understanding of the use of bifocal contact lenses for curbing myopia progression. By comparing the efficacy of high-add (+2.50D) and medium-add (+1.50D) options to single-vision contact lenses, researchers discovered that high-add lenses were more effective.
“This study showed that using multifocal contact lenses off-label for myopia control can provide effective slowing of myopia progression, as long as doctors use the highest add power available,” Dr. Walline notes, emphasizing that it opened up new options for myopia control, which is important since “no single treatment will work for all myopic children.”
Soft contact lenses are the top treatment for myopia management, according to Dr. Pucker, which underscores the ongoing relevance of the BLINK analysis. Dr. Tomiyama adds that while there are other multifocal lens options available, “this design is still used and is potentially more affordable for the patient—allowing greater access to myopia control.”
Looking ahead, Dr. Pucker highlights the need for further work to determine the best optical design. Dr. Tomiyama says that several papers are expected to be published in the near future, and one will address whether there is any evidence of rebound after discontinuing treatment.
MISIGHT MiSight Study. The MiSight lens from CooperVision, approved in 2019 and launched the following spring, remains the only soft lens indicated to slow myopia progression—a significant milestone.
This prospective study evaluated the efficacy of MiSight daily disposable soft lenses in slowing the progression of juvenile-onset myopia. These lenses were compared to conventional single-vision lenses over a three-year period. Results showed that MiSight slowed myopia progression 59% as measured by mean cycloplegic spherical equivalent and 52% as measured by mean axial elongation.
“The demonstrated efficacy was greater than what was shown in BLINK and reaches levels similar to other methods that have been considered ‘first-line,’ such as 0.05% atropine and ortho-K,” remarks Dr. Salzano. “More work needs to be done, but getting over 50% efficacy is an important achievement.”
The FDA approval of MiSight lenses changed the landscape of myopia management, giving both doctors and patients greater confidence in the modality, Dr. Salzano emphasizes. The MiSight study established a strong foundation for myopia management and the lens’s FDA approval allowed CooperVision to promote it to practitioners and the public, substantially raising awareness about the merits of myopia management.
 |
| The MiSight lens can slow progression of axial elongation by 52% and refractive error increase by 59%, its pivotal trial found. Photo: CooperVision. Click image to enlarge. |
CHAMP Childhood Atropine for Myopia Progression. Another atropine study, CHAMP evaluated preservative-free 0.01% and 0.02% options in slowing myopia progression. Results garnered a mixed reception.
Dr. Tomiyama notes that “0.01% was shown to be effective—more than 0.02%—which was surprising.”
However, neither option really separated from placebo in a significant way. Low-dose atropine “has long been hypothesized to reduce myopia progression with minimal side effects,” notes Dr. Hindman. “Unfortunately, the rate of placebo response was not much less than the 0.01% and nearly equivalent to the 0.02%.” Based on these findings, the FDA did not accept a New Drug Application for this drug; however, the developer is generating a response and approval could still be forthcoming, she notes. Several other companies are also conducting their own trials with low-dose atropine.
“Currently, we can prescribe low-dose atropine, but it is off-label, needs to be compounded at a pharmacy and is not covered by insurance,” Dr. Hindman explains. “All of these things make prescribing low-dose atropine less attractive than some other myopia control options available at this time.”
As research continues, our understanding of the usefulness of this approach will continue to grow. Dr. Tomiyama suggests future investigations may evaluate the long-term efficacy and explore different administration schedules or the need for tapering. Dr. Hindman notes the importance of assessing for a rebound effect after cessation of atropine, as some studies have indicated this could be a concern.
MTS Myopia Treatment Study. This randomized trial also compared low-dose 0.01% atropine drops to placebo for the slowing of myopia progression in children.
Reporting on the study’s key finding, Dr. Walline notes, “This study showed that 0.01% atropine did not slow myopia progression or eye growth compared to placebo over a two-year period,” challenging the viability of this strategy for myopia management.
“When coupled with the CHAMP and MOSAIC results, many practitioners have stopped using 0.01% atropine in their patients, at least as monotherapy,” adds Dr. Salzano. “It also speaks to the continued need for control groups, because if there was not a consistent control group for this study, it may have taken much longer to determine that 0.01% had a clinically minimal effect.”
The MTS was well-designed with a robust follow up, which only strengthens its significance, suggests Dr. Salzano. “It is very relevant as it builds on the literature that shows little to no effect of 0.01% atropine in a North American population.”
Research is currently underway exploring alternative formulations and dosages. Although it remains to be seen whether low-dose atropine can have a clinically meaningful effect in North American children, emerging data from European studies may provide new insights, Dr. Salzano notes.
Pediatric Vision Care
ATS Amblyopia Treatment Studies. Almost 30 years ago, a group of pediatric ophthalmologists and optometrists formed the Pediatric Eye Disease Investigator Group (PEDIG) and initiated a series of studies on childhood eye disorders, performing groundbreaking work on strabismus and amblyopia (as well as other topics). Papers on amblyopia began appearing around 2002 and have been released at regular intervals ever since. The studies look into various treatment modalities alone or in combination—including patching, atropine drops and glasses—and answered several important questions about amblyopia and its management.
 |
| One key goal of the Amblyopia Treatment Studies is to determine the optimal regimen of patch therapy for clinicians to recommend. Photo: NIH. Click image to enlarge. |
Data showed that spectacles alone are a reasonable initial treatment, Dr. Salzano notes, and highlights other key takeaways from these studies: two hours of patching is equivalent to six hours among mild-to-moderate amblyopes while six hours is equivalent to full-time patching in those with severe cases. The findings also demonstrated the comparable efficacy of biweekly 1% atropine to patching. “They also gave numerous data points such as an expected timeline for patching to plateau, an expected timeline for acuity improvement in bilateral refractive amblyopes, proof that amblyopia treatment could be successful (albeit more difficult) in older children and the importance of tapering patching to two hours a day prior to discontinuing in order to prevent recurrence,” Dr. Salzano explains.
This work also led to the field’s understanding that “amblyopia can be treated beyond the previously understood critical period of seven years,” says Brenda Montecalvo, OD, who practices at Nova Vision Care in Beavercreek, OH.
The Amblyopia Treatment Studies have led to changes in clinical practice. Today, spectacle lenses are commonly used as an initial treatment, with patching or atropine introduced later, which may reduce the treatment burden for patients, explains Dr. Salzano. While other methods are under investigation, these studies refined the use of spectacles, patching and atropine, which remain the standard of care for initial treatment of amblyopia, Dr. Salzano states.
There are a number of research avenues that warrant further investigation in ongoing and future studies to build on the ATS foundation. For example, Dr. Montecalvo notes the value of determining the “optimum vision therapy techniques that should be used to achieve high levels of binocularity and normal 20/20 eyesight.” Research efforts are also needed to develop faster training approaches that are both effective and safe, she adds. Some of the newest ATS research has explored the merits of interactive video games, perceptual learning (a new technique that may be superior to monocular methods) and dichoptic training (presenting different stimuli to each eye simultaneously).
Beyond ATS, other research on strabismus also showed that binocular treatments yield better vision outcomes, and alternative options like vision therapy and medication can be as effective as patching, says Dr. Montecalvo.
CITT Convergence Insufficiency Treatment Trial. According to Dr. Montecalvo, “This study confirmed the long-held clinical impression that in-office vision therapy was the most effective method in the treatment of convergence insufficiency” when compared with placebo, home-based vision therapy and pencil push-ups. CITT also showed that prism glasses weren’t more effective than placebo, says Dr. Salzano.
 |
|
The CITT-ART trial validated the benefits of in-office vision therapy for certain binocular vision disorders in children. Photo: Brenda Montecalvo, OD. Click image to enlarge. |
Following CITT, “vision therapy gained broader acceptance as the preferred treatment for convergence insufficiency,” notes Dr. Salzano, while highlighting the clinical implications of this research. Additionally, intraprofessional referral and collaboration between primary care optometrists and ODs specializing in vision therapy has increased in its wake. Dr. Montecalvo emphasizes a growing awareness that convergence insufficiency is both measurable and treatable.
Continued research has validated these results, although there are still questions surrounding impact on reading and learning, which have been explored to some extent by CITT-ART, Dr. Salzano shares.
Dr. Montecalvo recommends further research to investigate the correlation between academic performance and convergence insufficiency (CI), examine its relationship with athletic performance and compare the effectiveness of lens prescriptions to other treatments for kids with limited access to in-office therapy. Additionally, exploring why some individuals with CI excel academically while others do not could offer valuable insights.
VIP-HIP Hyperopia in Preschoolers Study. This work, which sought to determine the impact of hyperopia on early literacy and visual function in preschool children, had a major impact on clinical practice, offering valuable insights and informing evidence-based screening guidelines.
“VIP-HIP showed uncorrected moderate hyperopia was associated with reduced near function, reduced early literacy and attention problems when compared with emmetropic patients,” says Dr. Salzano, while Dr. Montecalvo emphasizes that hyperopic spectacle correction “improves children’s cognitive and educational well-being, psychological well-being, mental health and quality of life.”
Not only did this study help improve screening guidelines across the US, and in turn public health, it also helped practitioners understand the importance of correcting hyperopia, especially when near visual performance is reduced, Dr. Salzano explains.
Given the importance of screening guidance to ensure children receive the care they need, this analysis remains highly relevant today, according to Dr. Salzano, who notes that VIP-HIP has been instrumental in establishing current best practices. Dr. Montecalvo adds that with the growing use of digital devices in educational settings, this research is even more critical, as children’s visual systems are increasingly focused on near tasks for extended periods.
Dr. Salzano suggests the logical next step is to determine whether correcting hyperopia can prevent or improve deficits in early literacy and development, potentially leading to more uniform prescribing practices among eyecare providers.
More to ComeResearch, by its very nature, never ends. The studies detailed here, as signficant as they are, will be built upon and eventually elipsed in importance. We at Review of Optometry pledge to enhance this research guide on our website with regular updates, adding write-ups of studies not included at press time as well as incorporating new interpretations of the studies described here as thinking evolves over time. |
IATS Infant Aphakia Treatment Study. This investigation explored the management of unilateral congenital cataract in infants, comparing the outcomes of intraocular lens (IOL) implantation and contact lens correction.
“This study helped to clarify the optimal timing of cataract surgery in infants, risk of adverse events when corrected with an IOL or aphakia on initial surgery and provided data on the expected outcome for acuity and stereopsis throughout childhood,” states Dr. Salzano.
Data revealed that while early intervention can improve the quality of vision, it also increases the risk of complications. Dr. Montecalvo notes, “Children in either group receiving primary intraocular lens implantation did not have a better visual outcome than those managed with conventional treatment.”
The IATS established clear outcome expectations, enabling providers to better communicate potential risks and benefits, Dr. Salzano explains. “IOL implantation should be considered in children who require cataract surgery after age seven months,” Dr. Montecalvo recommends. “IOL implantation for unilateral or bilateral cataract is the standard of care for children older than 24 months.”
With respect to future research, Dr. Salzano suggests more work is required to improve outcomes for these children, both in reducing adverse events and enhancing vision throughout their lifespans. “We know contact lenses and IOLs are both reasonable options, but objectives like better prediction of IOL power for future refractive error and more efficient regimens for amblyopia treatment could build on this study.”
CONTRIBUTORSRami Aboumourad, OD: Dr. Aboumourad currently practices at Bascom Palmer Eye Institute in Miami. Sherry Bass, OD: Dr. Bass is a distinguished teaching professor at the SUNY College of Optometry and an attending in the Retina and Electrodiagnostic Clinics in the University Eye Center. Brian Chou, OD: Dr. Chou practices in San Diego at ReVision Optometry, a referral-based clinic for keratoconus and scleral lenses. Jennifer P. Craig, PhD: Dr. Craig is a professor in the department of ophthalmology at the University of Auckland in New Zealand, where she heads the Ocular Surface Laboratory. Natasha Hindman, OD: Dr. Hindman is director of clinical research at Scott and Christie Eyecare Associates in the Pittsburgh area. Lyndon Jones, PhD: Dr. Jones is a professor at the School of Optometry and Vision Science and director of the Centre for Ocular Research & Education at the University of Waterloo in Ontario, Canada. Paul Karpecki, OD: Dr. Karpecki is director of cornea and external disease at the Kentucky Eye Institute in Lexington, KY. Kelly Malloy, OD: Dr. Malloy is a professor and the director of the neuro-ophthalmic disease service at Pennsylvania College of Optometry at Salus University in Philadelphia. Brenda Montecalvo, OD: Dr. Montecalvo practices at Nova Vision Care in Beavercreek, OH. She is a fellow of the College of Optometrists in Vision Development, the American Academy of Optometry and the College of Syntonic Optometry. Marc Myers, OD: Dr. Myers is an optometrist at the Coatesville Veterans Affairs Medical Center in Coatesville, PA. Kelly Nichols, OD: Dean of the University of Alabama at Birmingham School of Optometry. Andrew Pucker, OD, PhD: Dr. Pucker is the executive director of clinical and medical sciences at Lexitas Pharma Services. His independent research and clinical career have focused on dry eye, contact lenses and myopia development. Andrew Rixon, OD: Dr. Rixon is an attending optometrist at the Memphis Veteran Affairs Medical Center, a diplomate in glaucoma through the American Academy of Optometry and member of the Optometric Glaucoma Society. Aaron Salzano, OD: Dr. Salzano is an assistant professor at Pacific University College of Optometry in Forest Grove, OR. He participates in clinical research with the Pediatric Eye Disease Investigator Group (PEDIG). Joseph Shovlin, OD: Dr. Shovlin, a senior optometrist at Northeastern Eye Institute in Scranton, PA, is a fellow and past president of the American Academy of Optometry. Joseph Sowka, OD: Dr. Sowka is an attending optometric physician at Center for Sight/ US Eye in Venice, FL, where he focuses on glaucoma management and neuro-ophthalmic disease. Brad Sutton, OD: Dr. Sutton is a clinical professor at Indiana University School of Optometry and service chief of the Indianapolis Eye Care Center. Erin Tomiyama, OD, PhD: Dr. Tomiyama is an assistant professor of optometry at Marshall B. Ketchum University. She teaches in the basic science and contact lens curriculum, and spearheads the university’s myopia management service. Jeff Walline, OD, PhD: Dr. Walline is the acting dean and a distinguished professor at Ohio State University College of Optometry. He is also the president of the American Academy of Optometry. Sara Weidmayer, OD: Dr. Weidmayer practices at the Ltc. Charles Kettles VA Medical Center in Ann Arbor, MI. She is also a clinical assistant professor for the department of ophthalmology and visual sciences of the University of Michigan. James S. Wolffsohn, PhD: Dr. Wolffsohn is a professor at the School of Optometry and Vision Science, College of Health and Life Science at Aston University in the United Kingdom. |
1. Gurwood AS. The National Eye Institute of The National Institutes of Health: Its history and impact on clinical eye care. Optometry. 2003;74(10):627-57. 2. Heijl A, Peters D, Bengtsson B. Long-term impact of immediate versus delayed treatment of early glaucoma: results from the Early Manifest Glaucoma Trial. Am J Ophthalmol. 2023;252:286-94). 3. Cabrera MT, Chen A. It’s time we reform our perspectives on race and glaucoma. Transl Vis Sci Technol. 2022;11(9):22. 4. Siegfried CJ, Shui YB. Racial disparities in glaucoma: from epidemiology to pathophysiology. Mo Med. 2022;119(1):49-54. 5. King AJ, Hudson, J, Azuara-Blanco A, et al. Evaluating primary treatment for people with advanced glaucoma. Ophthalmology. 2024;131(7):759-70. 6. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Laser in glaucoma and ocular hypertension (LiGHT) trial: six-year results of primary selective laser trabeculoplasty versus eye drops for the treatment of glaucoma and ocular hypertension. Ophthalmology. 2023;130(2):139-51. 7. New York University Langone Health. Long-term low-dose antiviral treatment benefits patients with eye disease pain from shingles. NYU Langone Health. Oct 19, 2024. https://nyulangone.org/news/long-term-low-dose-antiviral-treatment-benefits-patients-eye-disease-pain-shingles. 8. Green M, Hughes I, Hogden J, Sara S, Apel A, Stapleton F. High-dose steroid treatment of bacterial keratitis. Cornea. 2019;38(2):135-40. 9. Hussain M, Shtein RM, Pistilli M, Maguire MG, Oydanich M, Asbell PA. The dry eye assessment and management (DREAM) extension study – a randomized clinical trial of withdrawal of supplementation with omega-3 fatty acid in patients with dry eye disease. Ocul Surf. 2020;18(1):47-55. 10. Brown DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: The 36-month results from two phase III trials. Ophthalmology. 2013;120(10):2022. 11. Heier JS, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2016;123(11):2376-85. 12. Standardization of Uveitis Nomenclature (SUN) Working Group. Development of classification criteria for the uveitides. Am J Ophthalmol. 2021;228:96-105. 13. MacLaren RE, Audo I, Fischer MD, et al. An open-label phase II study assessing the safety of bilateral, sequential administration of retinal gene therapy in participants with choroideremia: The GEMINI study. Hum Gene Ther. 2024;35(15-16):564-575. |