 |
The Tear Film and Ocular Surface Society (TFOS) is an organization that provides incredible insights, research, knowledge-sharing and camaraderie among dry eye disease (DED) experts around the world. Its 8th International Conference, held September 2016 in Montpellier, France, was another exceptional educational event. Here are a few abstract highlights from papers, posters and presentations:
Contact Lenses and MGD
Several presentations and papers, and a session comoderated by me and Donald Korb, OD, showed contact lenses likely contribute to structural changes within the meibomian gland framework, leading to meibomian gland dysfunction (MGD). Video imaging shows contact lens movement along the lower eyelid in the direction of the meibomian glands during a normal blink.1 Researchers postulate this friction over time may play a role.1 The research also shows a statistically greater amount of meibomian gland loss in contact lens wearers compared with an age-matched control group of non-wearers.1
Eric Papas, OD, conceded that contact lenses may indeed result in structural changes to the meibomian glands, but no study has yet confirmed functional changes directly associated with contact lens wear.1
All of this evidence (or lack thereof) suggests we should be monitoring our contact lens patients for structural changes with tests such as meibography throughout their lens wearing life and, once these changes are noted, take steps to educate and treat to minimize functional meibomian gland issues, contact lens drop-out and, potentially, chronic DED.
 |
| New research is helping to reshape dry eye screening and diagnosis. |
Diabetes and Dry Eye
Research showed a clear correlation between MGD and patients with Type 2 diabetes.2 One study found patients with Type 2 diabetes had a significantly lower noninvasive tear break-up time, higher levels of inflammation and obstruction of the meibomian glands. In addition, the lipid layer thickness was considerably lower than in normal subjects, resulting in increased subjective symptoms and a decreased quality of life.2 Another study showed the HbA1c level had a high degree of correlation with the symptoms based on the National Eye Institute Vision Functioning Questionnaire.3
Fish Oil, Krill Oil and DED
A recent clinical trial showed that omega fatty acid supplements are a valuable treatment option for DED. Researchers from the University of Melbourne in Australia showed a statistically significant improvement in osmolarity (p<0.001) in both phospholipid and triglyceride forms of omega fatty acids when measured three months after the study subjects began taking omega-3 supplements. The results also showed increased tear stability and an improvement in subjective symptoms.4
Symptoms But No DED
One poster looked at Tearlab’s osmolarity test, particularly when normal readings occur in patients who present with classic symptoms of DED.5 The patients had normal measurements (in this case, 50 patients with both eyes measuring under 300 mOsmol/L and within 5 mOsmol/L between eyes) with a mean tear osmolarity of 293.33 mOsmol/L (+/- 6.70) and a mean difference of 0.94 mOsmol/L between eyes (+/- 3.18).
An alternate diagnosis was established in 100% of these cases. The most frequent diagnoses were allergic conjunctivitis (24%) and anterior blepharitis (24%), followed by epithelial basement membrane dystrophy (12%), keratoneuralgia (12%), contact lens intolerance (8%), conjunctivochalasis (8%), computer vision syndrome (6%) and trichiasis (6%). Twenty-two percent of patients had more than one diagnosis.5
In the past, if a patient presented with fluctuating vision and dry, gritty, red eyes, doctors often initiated dry eye treatment, resulting in patient and doctor frustration when DED wasn’t the appropriate diagnosis. This research suggests normal osmolarity may be beneficial in establishing a differential diagnosis away from DED, even with classic DED symptoms.
Sex and the Eye
Though we have long known women are at a higher risk for DED, new developments led by David Sullivan, PhD, are adding to our body of knowledge. For one, his research reveals that the sex hormone receptors for estrogens and androgens are different between men and women.6 Because sex hormones can mediate inflammation—among other activities—in the eye, this research may explain why some therapies work and others don’t between the sexes, despite similar signs and symptoms.
Furthermore, testosterone deficiency can result in a significant alteration to the glycocalyx make-up on the ocular surface, causing ocular surface desiccation.6
We can’t forget the fact that, although DED is multifactorial, meibomian and lacrimal glands play a key role, and the cornea can be the most affected. New research suggests testosterone may also play a key role in corneal protection.7,8
Restoring Clarity to the Ocular Surface
Mesenchymal stem cells (MSC) may be the key to restoring transparency in a cornea after ocular injury, a new study suggests. Research shows that MSCs inhibit stromal fibrosis and secrete elevated levels of hepatocyte growth factor, resulting in decreased inflammation.9
Wrong Diagnosis for Millions
One of the great things about a symposium such as TFOS is that it leaves you with as many new questions as it does answers. As an example, leave it to Dr. Korb to question the common assumption that DED is under-diagnosed. And I have to agree with him after hearing the presentation; too many times we make a diagnosis based on symptoms that sound like dry eye disease. Or we make the assumption that patients with classic DED need to have treatments that focus on aqueous production and dry eye sequelae, when in fact more than 50% of these patients actually improve significantly from a treatment for MGD.10 After Dr. Korb’s presentation, I had to admit that I, too, believe MGD needs to be addressed in all patients with DED. This shift changes how we think about the disease, and although MGD may be a leading cause of DED, the two are not one and the same.
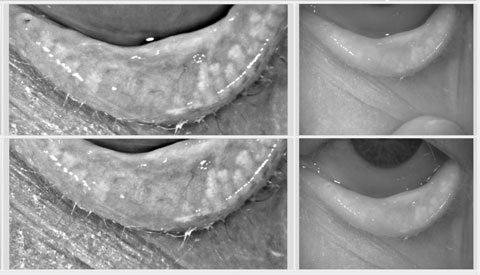 |
| Meibography can reveal functional meibomian glands, even when none are observable to the naked eye. |
No Observable Glands, But Treatment Isn’t Futile
Another poster by Dr. Korb and Caroline Blackie, PhD, OD, looked at meibography of the lower nasal eyelid and compared patients with no observable glands with a control group that had roughly five observable glands in the same area.11 When they tested this area they found that the dry eye patients still had a mean of 1.8 (+/- 1.1) functional glands, even though they were not observable.11 This dispels the myth that treatment is futile when no glands are seen. In fact, patients with this much structural damage are precisely the patients who require the most aggressive treatment to preserve, and possibly enhance, what little structure remains.
This is only a small sampling of the abstracts, posters and presentations provided at the annual meeting. TFOS is a terrific organization to join if dry eye and ocular surface disease is an important aspect of your practice. It’s a valuable resource for researchers and clinicians alike with insights that can be deep into immunology while also extremely practical and clinical. All of the new research it provided clearly shows we have come a long way in the understanding of DED—but we still have a long way to go before we fully understand this complex and multifactorial disease.
|
1. Arita R. Do contact lenses cause clinically relevant meibomian gland dysfunction? Prime Time TFOS Debates 2 presented at the 8th International Conference on the Tear Film & Ocular Surface (TFOS), September 8, 2016; Le Corum, Montpellier, France. 2. Garzon Johanna P, Lopez-Alemany A. Correlation between tear film lipid layer by interferometry and symptoms in diabetic patients with meiboiman gland dysfunction. Poster presented at the 8th International TFOS Conference. 3. Garzon Johanna P, Lopez-Alemany A. Clinical features of meibomian gland dysfunction in patients with diabetes Type 2. Poster presented at the 8th International TFOS Conference. 4. Downie LE, Deinema L, Chinnery HR, Vingrys AJ. A randomized, double-masked, placebo-controlled, clinical trial of two forms of omega-3 supplements for treating dry eye disease. Poster presented at the 8th International TFOS Conference. 5. Brissette AR, Bohm KJ, Starr CE. The utility of a normal tear osmolarity test in symptomatic patients. Poster presented at the 8th International TFOS Conference. 6. Sullivan DA. Liu Y, Ding J, Kam WR. Ménage à trois: sex, sex steroids and dry eye disease. Keynote address presented at the 8th International TFOS Conference. 7. Kadmiel M, Cidlowski JA. Glucocorticoids, sex and inflammation. Keynote address presented at the 8th International TFOS Conference. 8. Clayton J. Studying both sexes: a guiding principle for ophthalmology. Presented at the 8th International Conference on the TFOS. 9. Chauhan S, Restoration of corneal transparency by mesenchymal stem cells. Keynote address presented at the 8th International TFOS Conference. 10. Korb DR. Blackie CA. Is dry eye the wrong diagnosis for millions? Poster presented at the 8th International TFOS Conference. 11. Korb DR. Blackie CA. Can meibography fail to reveal functional gland structure? Poster presented at the 8th International TFOS Conference. |

