 |
A 78-year-old Hispanic male presented for a new patient exam following uncomplicated cataract surgery 12 months prior; he had no visual complaints. Past medical history included coronary artery disease and stage IV metastatic lung cancer diagnosed 10 years prior. He received numerous rounds of chemotherapy and radiation since diagnosis and is still undergoing active treatment with pembrolizumab, docetaxel and ramucirumab under the care of his oncology team.
Best-corrected visual acuity was 20/25+2 OD and 20/20 OS, intraocular pressure was 11mm Hg OD and 10mm Hg OS, pupils were equally round and reactive without a relative afferent pupillary defect, extraocular motilities were full in all gazes and confrontation visual fields were full to finger counting in both eyes. Slit lamp examination revealed well-positioned intraocular lenses OU and trace posterior capsular opacification OD.
 |
|
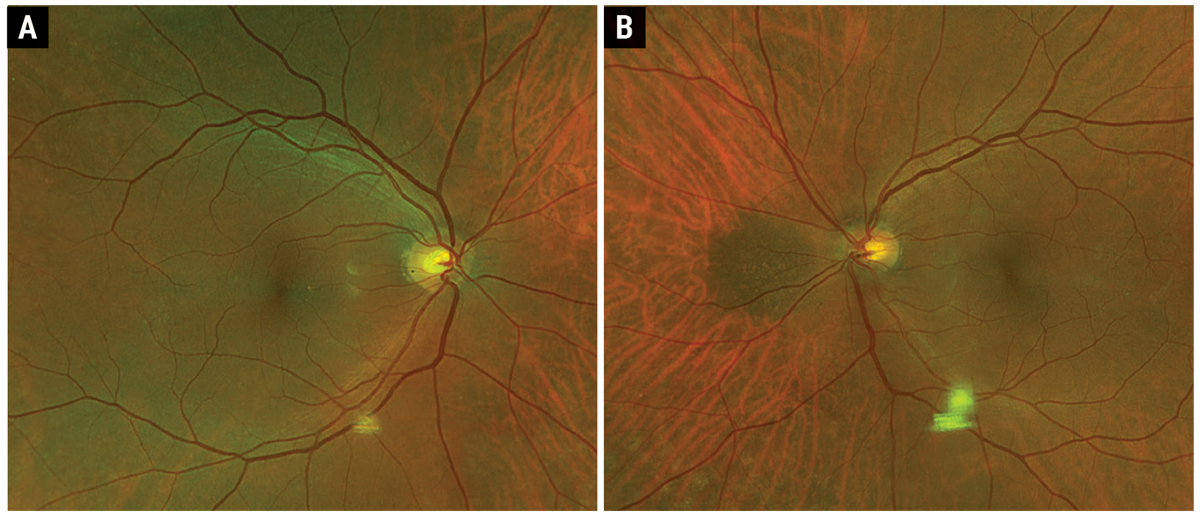
Fig. 1. Optos fundus photo of the right (A) and left (B) eye. Click image to enlarge. |
Take the Retina Quiz
1. What is the most likely diagnosis?
a. Choroidal melanoma.
b. Choroidal metastasis.
c. Choroidal nevus.
d. Congenital hypertrophy of the RPE.
2. Which of the following statements is true regarding this lesion?
a. Already malignant with high risk of metastasis.
b. Already malignant with low risk of metastasis.
c. Benign with high risk of transformation.
d. Benign with low risk of transformation.
3. Which of the following clinical findings is a risk for malignancy?
a. 3mm basal diameter.
b. 3mm thickness.
c. Thinning of the overlying retina.
d. Presence of drusen.
4. Which of the following imaging modalities is frequently used to assess this condition?
a. B-scan ultrasound.
b. OCT.
c. Fundus autofluorescence (FAF).
d. All of the above.
5. What is the most appropriate management of this patient?
a. Enucleation.
b. Fine needle aspiration biopsy.
c. Plaque brachytherapy.
d. Repeat examination in six months.
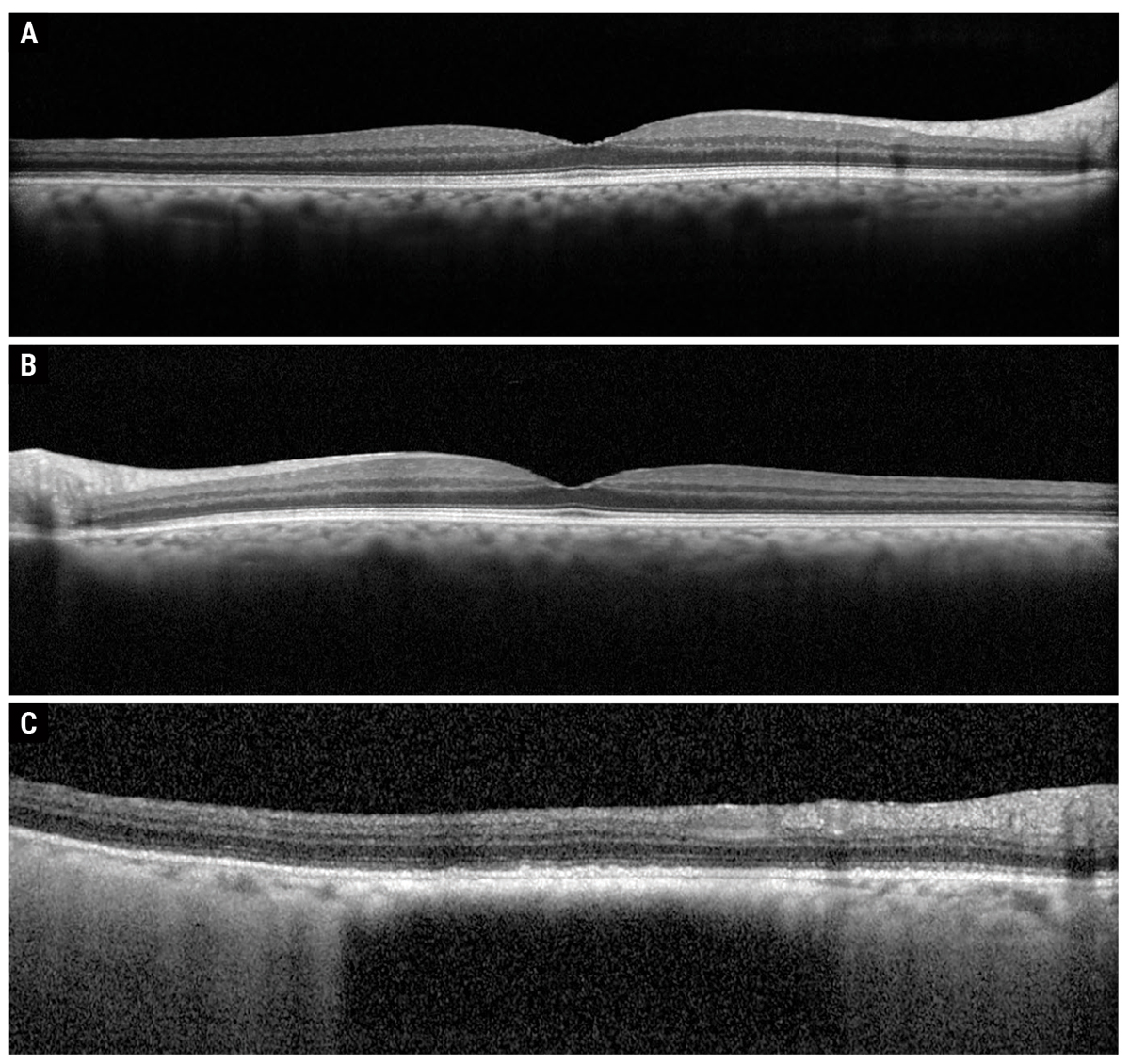 |
| Fig. 2. Heidelberg OCT of the right macula (A), left macula (B) and peripapillary lesion (C). Click image to enlarge. |
Diagnosis
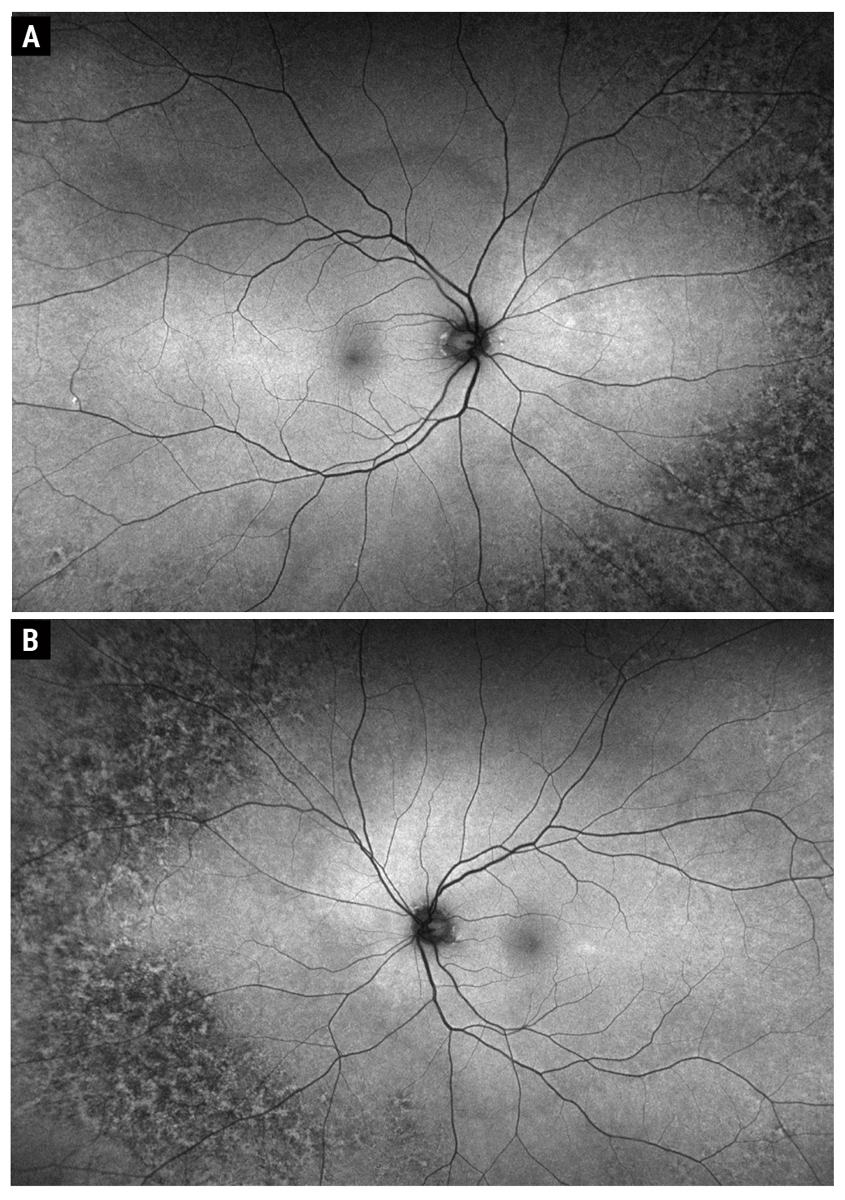
Fundus examination showed peripheral reticular degeneration OU and a 2.5-disc diameter hyperpigmented peripapillary lesion. The lesion was flat with overlying drusen, but no orange pigment or subretinal fluid (Figures 1A and B). OCT showed that the macula was flat OU (Figures 2A and B). OCT through the lesion showed homogeneous choroidal hyperreflectivity with overlying subretinal pigment epithelial (RPE) deposits and confirmed that the lesion was flat with no associated subretinal fluid; the lesion measured 3mm at greatest basal diameter on the OCT module (Figure 2C). FAF showed peripheral reticular degeneration OU without any abnormal peripapillary autofluorescence (Figures 3A and B).
Discussion
Choroidal nevi are the most common primary intraocular tumors and are benign in nature.1 There is no gender predilection, and prevalence is estimated to be 5% to 25%, though highly variable based on the sampled population demographic.1-3 For example, prevalence is estimated to be 5.6% in a Caucasian population, 2.7% in a Hispanic population and 2.1% in a Black population; however, the overall estimated prevalence of choroidal nevi in adults over age 40 in the United States is 4.7%.2,3
A series of 3,806 patients showed that 95% were Caucasian with a median age of 62.5, with the odds of having a choroidal nevus being 10-times higher in Caucasians and five-times higher in Hispanic vs.Black patients.3,4
Clinical Features
Choroidal nevi are commonly found as incidental findings on routine fundoscopy. They are typically well-circumscribed choroidal lesions <2mm in thickness and <5mm in diameter.1 They may be melanocytic (pigmented) or amelanotic (nonpigmented).1 Although nevi are benign, there exists an inherent risk of transformation to melanoma which carries with it the possibility of associated morbidity and mortality via metastasis.1
 |
|
Fig. 3. Optos fundus autofluorescence of the right (A) and left (B) eye. Click image to enlarge. |
Clinical Assessment of Risk Factors
One study extensively published on the topic of choroidal nevus and established the mnemonic we now use routinely when assessing choroidal nevi to stratify risk of malignant transformation: To Find Small Ocular Melanoma-Using Helpful Hints Daily (TFSOM-UHHD).5,6 Clinical risk factors for malignant transformation include: thickness >2mm; presence of subretinal fluid; presence of symptoms; presence of orange pigment (lipofuscin); posterior tumor margin <3mm from optic disc; ultrasound hollowness; absence of depigmented halo surrounding lesion; absence of drusen.5-7 Low-risk clinical features include the presence of overlying drusen or RPE alterations, suggesting chronicity and representing overlying degenerative changes.1
In 1995, another study found that the clinical features of small melanocytic choroidal lesions portending greatest risk of metastatic disease were tumor location (posterior margin touching the optic disc), increased tumor thickness, symptomatic blurry vision and documented tumor growth.6 However, the authors refuted this more recently in a 2019 analysis that showed proximity to disc, absence of drusen and absence of depigmented halo were not significant.4 The aforementioned risk factors are actually most likely indicative of tumors that have already undergone malignant transformation rather than factors that precede it.6 Documented growth carries an eight-times greater risk of metastatic disease than non-growing tumors.6
The mnemonic was therefore revised in 2019 to reflect lesion diameter: To Find Small Ocular Melanoma Doing Imaging (TFSOM-DIM).4 The advent of multimodal imaging has further refined our ability to structurally assess these lesions in greater detail. OCT is excellent for identifying and monitoring drusen progression and overlying RPE changes, and it can also demonstrate overlying thinning and cystoid degenerative changes of the retina, which suggest chronicity.4 The clinical finding of orange pigment is well-demonstrated as hyperAF on FAF imaging.4 B-scan ultrasound is useful for detecting low echogenicity (hollowness) but is limited in its utility for thinner tumors.4,8
Enhanced-depth imaging (EDI) spectral-domain OCT allows for improved imaging capability of the choroid and provides an axial resolution of approximately 3µm to 4µm compared to 50µm to 200µm with ultrasound imaging.9 Ultrasound tends to overestimate tumor thickness for flatter lesions, which is likely due to the imprecision associated with lower resolution and inability to accurately distinguish the junction of the overlying retina and underlying sclera.9 However, limitations of OCT include patient cooperation, degree of lesion pigmentation, media clarity and anterior location.8 In general, EDI-OCT is perhaps more useful and sensitive at monitoring small, flat, posterior lesions than ultrasound and/or clinical exam alone.8
Management, Prognosis and Risk of Transformation
It is recommended that choroidal nevi be evaluated twice in the first year they are observed, followed by annual examinations if noted to be stable in the first year. Ophthalmic and systemic morbidity and mortality due to choroidal nevi typically occur in the presence of subretinal fluid, choroidal neovascularization and malignant transformation.2,4 In rare cases (~1%), choroidal nevi may grow exudative neovascular membranes that can require therapy.4 Furthermore, all nevi carry potential for malignant transformation and should be followed accordingly. Kaplan-Meier estimates showed that in five years, patients with zero to five risk factors carried a 1.1%, 11%, 22%, 34%, 51% and 55% risk, respectively, of malignant transformation.4
Our patient has been followed for two years without demonstrable change in lesion size, presence of subretinal fluid or development of orange pigment.
Retina Quiz Answers
1: c, 2: d, 3: b, 4: d, 5: d
Dr. Aboumourad currently practices at Bascom Palmer Eye Institute in Miami. He has no financial disclosures.
1. Shields CL, Lally SE, Dalvin LA, et al. White paper on ophthalmic imaging for choroidal nevus identification and transformation into melanoma. Transl Vis Sci Technol. 2021;10(2):24. 2. Chien JL, Sioufi K, Surakiatchanukul T, et al. Choroidal nevus: a review of prevalence, features, genetics, risks and outcomes. Curr Opin Ophthalmol. 2017;28(3):228-37. 3. Qiu M, Shields CL. Choroidal nevus in the United States adult population: racial disparities and associated factors in the National Health and Nutrition Examination Survey. Ophthalmology. 2015;122(10):2071-83. 4. Shields CL, Dalvin LA, Ancona-Lezama D, et al. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2,355 cases: The 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina. 2019;39(10):1840-51. 5. Shields CL, Furuta M, Berman EL, et al. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol. 2009;127(8):981-7. 6. Shields CL, Shields JA, Kiratli H, et al. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology. 1995;102(9):1351-61. 7. Shields CL, Kels JG, Shields JA. Melanoma of the eye: revealing hidden secrets, one at a time. Clin Dermatol. 2015;33(2):183-96. 8. Shields CL, Kaliki S, Rojanaporn D, et al. Enhanced depth imaging optical coherence tomography of small choroidal melanoma: comparison with choroidal nevus. Arch Ophthalmol. 2012;130(7):850-6. 9. Say EA, Shah SU, Ferenczy S, Shields CL. Optical coherence tomography of retinal and choroidal tumors. J Ophthalmol. 2012;2012:385058. |

