 |
A 71-year-old Hispanic female presented with blurry and distorted vision in her left eye for the past month. She had been seen 14 months earlier, and at that time her vision was 20/20. She has been moderately hyperopic all her life and felt like she always saw well with her glasses. The blur and distortion in the left eye represented a recent change. She suffers from dry eye and had been using artificial tears and Restasis (cyclosporine, Allergan) with moderate relief of her symptoms.
Her medical history is significant for hypertension, and she takes atenolol and hydrochlorothiazide.
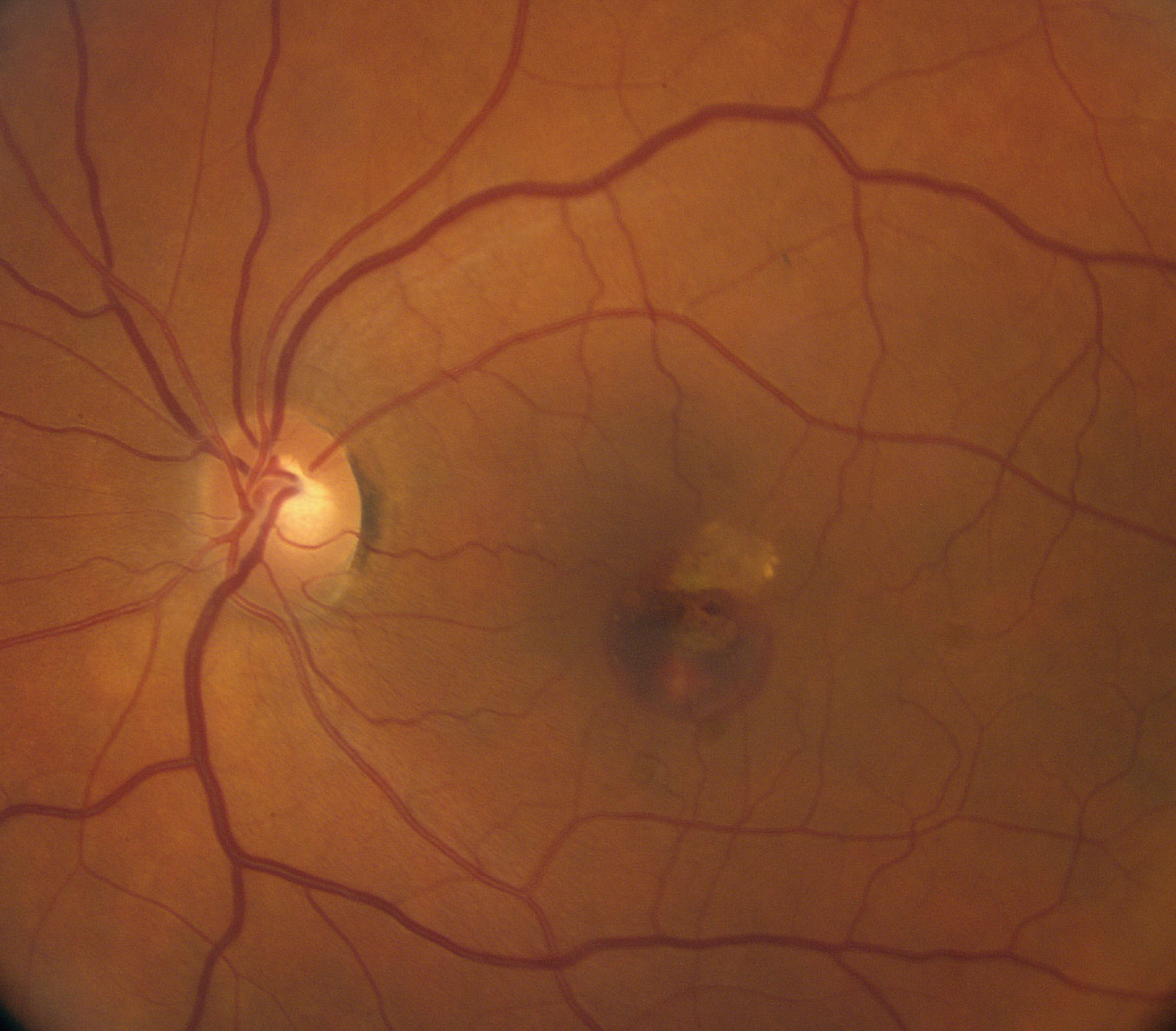 |
| Fig 1. This fundus photo shows our patient’s left eye. How do you account for the hemorrhage? Click image to enlarge. |
Evaluation
On examination, her best-corrected visual acuity was 20/20 OD and 20/70 OS. Her manifest refraction was +8.00 -3.00 x 070 OD and +6.25 -1.25 x 090 OS. Her confrontation visual fields were full-to-careful finger counting. Her ocular motility testing was normal and the pupils were equally round and reactive to light without an afferent pupillary defect. The anterior segment was significant for trace nuclear sclerotic cataracts in both eyes. Tensions by Tonopen (Reichert) measured 16mm Hg OU.
On dilated fundus exam, the vitreous was clear. The optic nerves were healthy with small cups and good rim perfusion in both eyes. The macula, vessels and periphery of the right eye were all normal. In the left, obvious changes in the macula were visible (Figure 1). Spectral-domain OCT (SD-OCT) and OCT angiography (OCT-A) scans were also obtained (Figures 2 and 3).
Take the Retina Quiz
1. What is responsible for the macular changes in the left eye?
a. Subretinal hemorrhage.
b. Suprachoroidal hemorrhage.
c. Choroidal neovascularization.
d. Polypoidal choroidal vessels.
2. What is the etiology?
a. Macular degeneration.
b. Myopic degeneration.
c. Idiopathic.
d. Pachychoroidal neovascularization.
3. How should this patient be managed?
a. Pars plana vitrectomy.
b. Anti-VEGF injections.
c. Photodynamic therapy.
d. Focal laser photocoagulation.
4. What additional testing would be helpful for this patient?
a. Visual field.
b. Ultrasound.
c. A-scan.
d. Enhanced-depth imaging OCT.
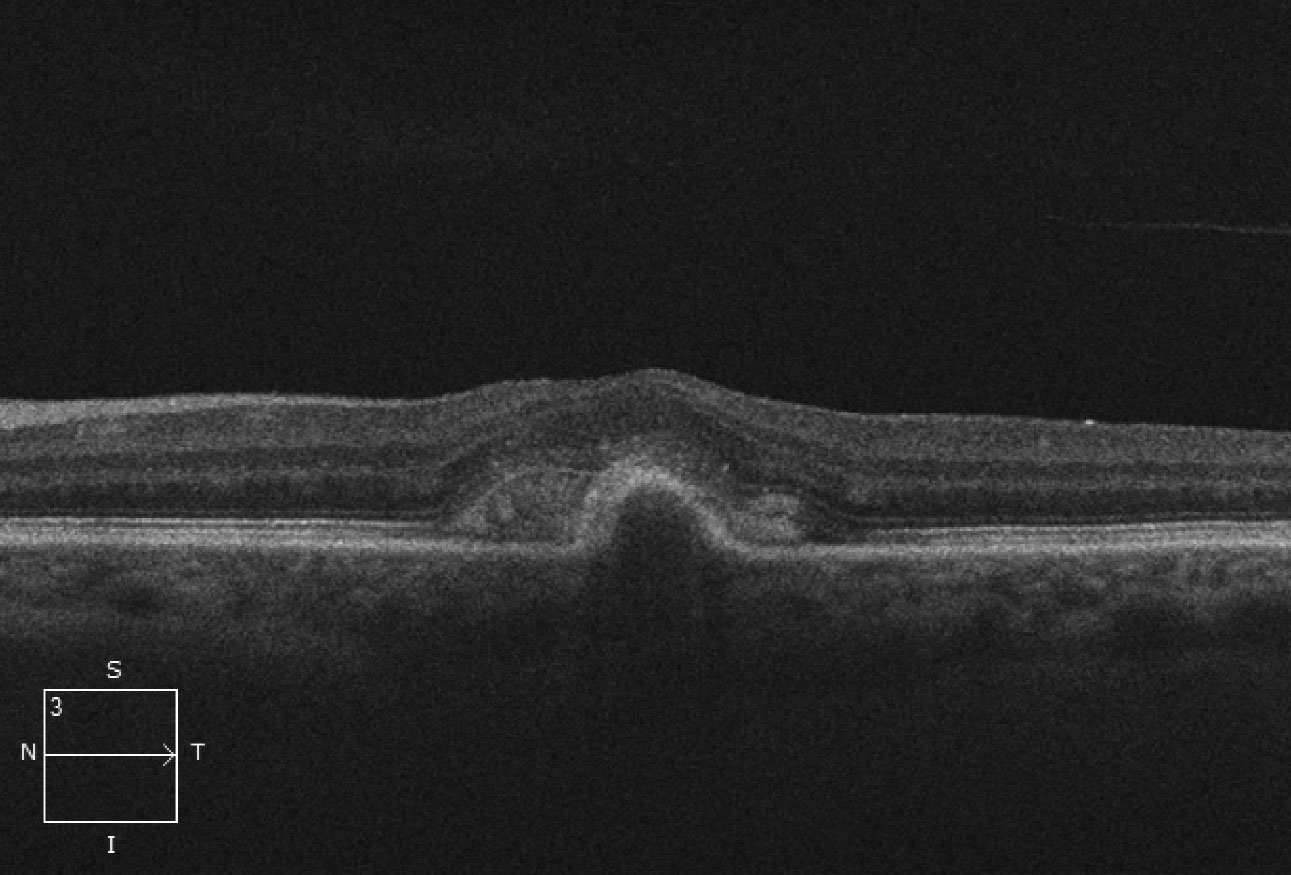 |
| Fig. 2. This OCT scan shows us what’s beneath the patient’s macula. Click image to enlarge. |
Diagnosis
The obvious subretinal hemorrhage in the macula of the left eye is due to choroidal neovascularization (CNV). The SD-OCT shows loss of the foveal depression and a serous pigment epithelial detachment (PED) involving the macula. Adjacent to the PED and above the retinal pigment epithelium (RPE) is a high reflective area that represents the CNV. This is a Type 2 CNV because it is growing above the RPE.
Part of the CNV can be visualized on the deep slice of the OCT-A scan. If we had a slightly deeper cut we would have been able to distinguish even more. The en face images show a large area of hyperfluoresence. The deeper avascular slice was essentially normal, which is consistent with the clinical presentation. Interestingly, no subretinal or intraretinal fluid is present.
Why did she develop this? The most common causes of CNV is macular degeneration, histoplasmosis and pathologic myopia. Other predisposing causes include angioid streaks, trauma and inflammation, among others. When no apparent cause can be detected, we assume the condition is idiopathic.
In our patient, no drusen or RPE mottling was noted in either eye, so that eliminates age-related macular degeneration as a cause—even though she fits the age demographic.
Of interest, she has had a longstanding circular area of RPE atrophy, like a scar, that can be seen superior to the macula that has been present for many years. This is probably the access point for developing the CNV.
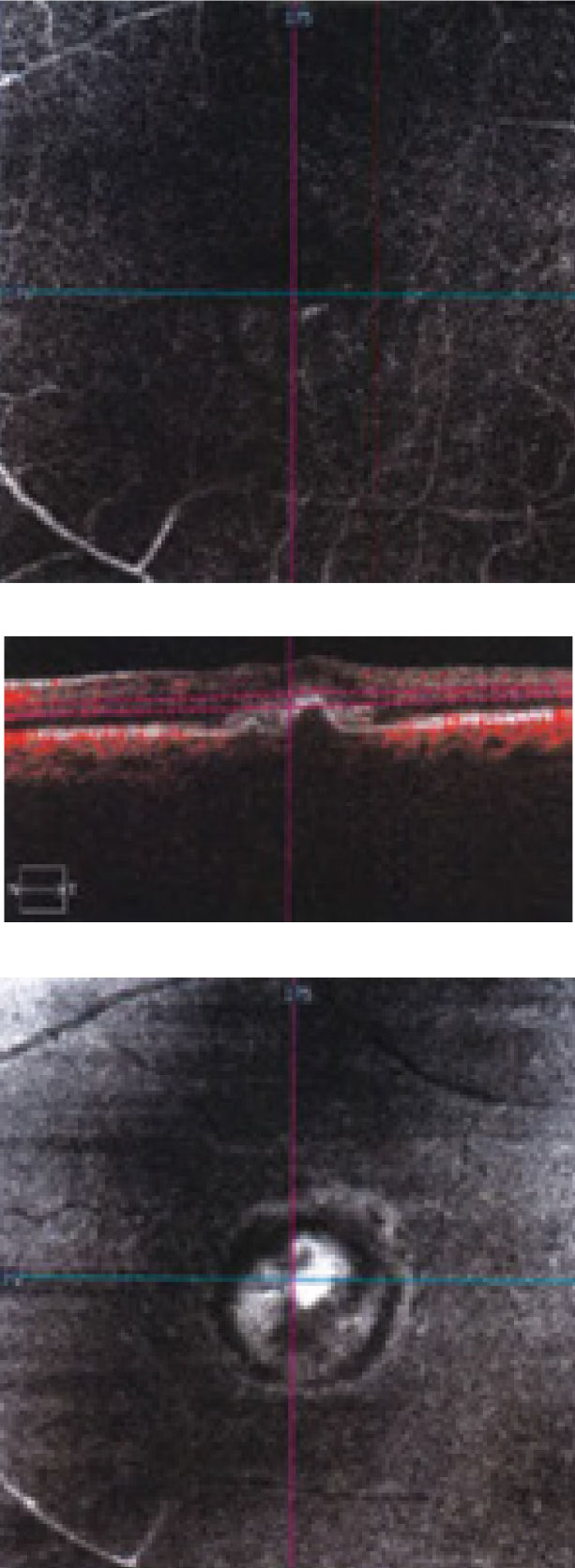 |
| Fig 3. This “deep slice” OCT-A shows an en face view of through the patient’s macula. Click image to enlarge. |
Discussion
CNV commonly grows adjacent to chorioretinal scars, which themselves develop following toxoplasmosis, laser photocoagulation and end-stage macular telangiectasia, among others. Any of these can result in a break in Bruch’s membrane, which sets the stage for CNV.
Our patient’s longstanding area of RPE atrophy above the fovea was seen in the fundus photograph, but it hadn’t resulted in any problems until the CNV developed.
Another possible cause of CNV is pachychoroid, which is characterized by choroidal thickening and RPE changes. Choroidal thickness varies with age, ethnicity and axial length. The normal choroidal thickness is between 191µm and 354µm. Patients with a choroidal thicknesses greater than 390µm have a pachychoroid.1-2
A spectrum of conditions are associated with this finding, including pachychoroid pigment epitheliopathy (PPE), central serous chorioretinopathy (CSCR), pachychoroid neovasculopathy (PNV) and polypoidal choroidal vasculopathy (PCV). PPE is considered the precursor to CSCR.1-2
Each of these conditions may progress to the development of neovascularization below the RPE as well as polypoidal choroidal vessels as seen with PCV. These patients may present with pigmentary changes, idiopathic serous PEDs and even hemorrhagic PEDs in more advanced cases.
Many patients who were once considered to have idiopathic CNV are now recognized as having PPE. Advances in OCT/OCT-A imaging including enhanced-depth imaging OCT, hasve led to a much better understanding of these diseases as well as more accuracy in making the diagnosis.
Our patient was referred to a retina specialist where she ultimately received five anti-VEGF injections over 10 months. The hemorrhage resolved and her foveal contour and architecture returned to normal. On last exam, her vision had improved to 20/40. She continues to be followed closely.
1. Yap J, Mandelcorn ED. Pachychoroid spectrum: a closure look. Retina Specialist. 2018;4(3):44-7. 2. Dolz-Marco R, Dansingani K, Freund K. The pachychoroid clinical spectrum, A new risk and disease modifier in macular disorders. Retina Today. May/June 2016;14(4):57-60. |
Retina Quiz Answers:
1) c; 2) c; 3) b; 4) d

