Annual Retina ReportCheck out the other feature articles in this month's report:Sizing Up Geographic Atrophy Macula Exam Tips and Tricks Reconsider Your Approach to Diabetic Retinopathy Tracing Clues Back to Retinitis Pigmentosa |
Intravitreal injections are the most commonly performed ophthalmologic procedure—more so even than cataract surgery—with over three million injections performed in 2016.1 With an aging population and an expected coinciding rise in retinal disease, studies suggest these numbers will only grow.2 One particular class, anti–vascular endothelial growth factor (VEGF) medications, can treat patients with various retinal conditions.
While optometrists don’t perform the procedure, patients value guidance from their ODs, and providing that guidance requires knowledge of how the procedure is performed, any complications that can arise and what types of patients benefit from these treatments. This helps to make appropriate referrals, manage co-existing ocular conditions, set patient expectations and identify potential side effects.
This article explains the procedure, changes its seen over the years and the academic literature that can help you communicate the key points to your patients.
Yes, a Needle In Your Eye
To patients who’ve never received an intravitreal injection, a needle to the eye can sound unnerving. They need reassurance that this is a common and low-risk procedure with a significantly positive outcome. Explain that, when they arrive at their ophthalmologist’s office, they will be asked to sign a consent form that discusses the risks, benefits and alternatives of intravitreal injection. Assure them their doctor will follow established protocols and explain that the majority of endophthalmitis complications stem from the practitioner’s or even the patient’s own oral flora—so, they should refrain from speaking during the procedure.3,4 Research shows a clean room in an office setting is adequate to reduce the risk of infection.5
Due to the increasing incidence of bilateral macular edema as well as bilateral choroidal neovascular membranes (CNVM), many patients now require bilateral injections.6 This can reduce the treatment burden, and it’s generally well tolerated, preferred by patients and appears safe, as no evidence shows bilateral injections increase the endophthalmitis risk.6
Assuage your patients’ fears by explaining that they will be given adequate anesthesia to minimize discomfort. While a number of studies compare various techniques, the literature shows no consistently preferred approach. Researchers looked at proparacaine, tetracaine, lidocaine pledgets or gel and subconjunctival injection of 2% lidocaine and found patients reported mild pain only, regardless of anesthesia used.7 Most clinicians opt for localized application of topical anesthetic with cotton pledgets, as it is easy and seems to work as well as other methods.7
Injections are commonly made 3.5mm from the limbus in pseudophakic eyes and 4.0mm in phakic eyes. Theoretically, the injection can be performed in any quadrant of eye, but is typically done in the superotemporal or inferotemporal quadrant due to ease of access.
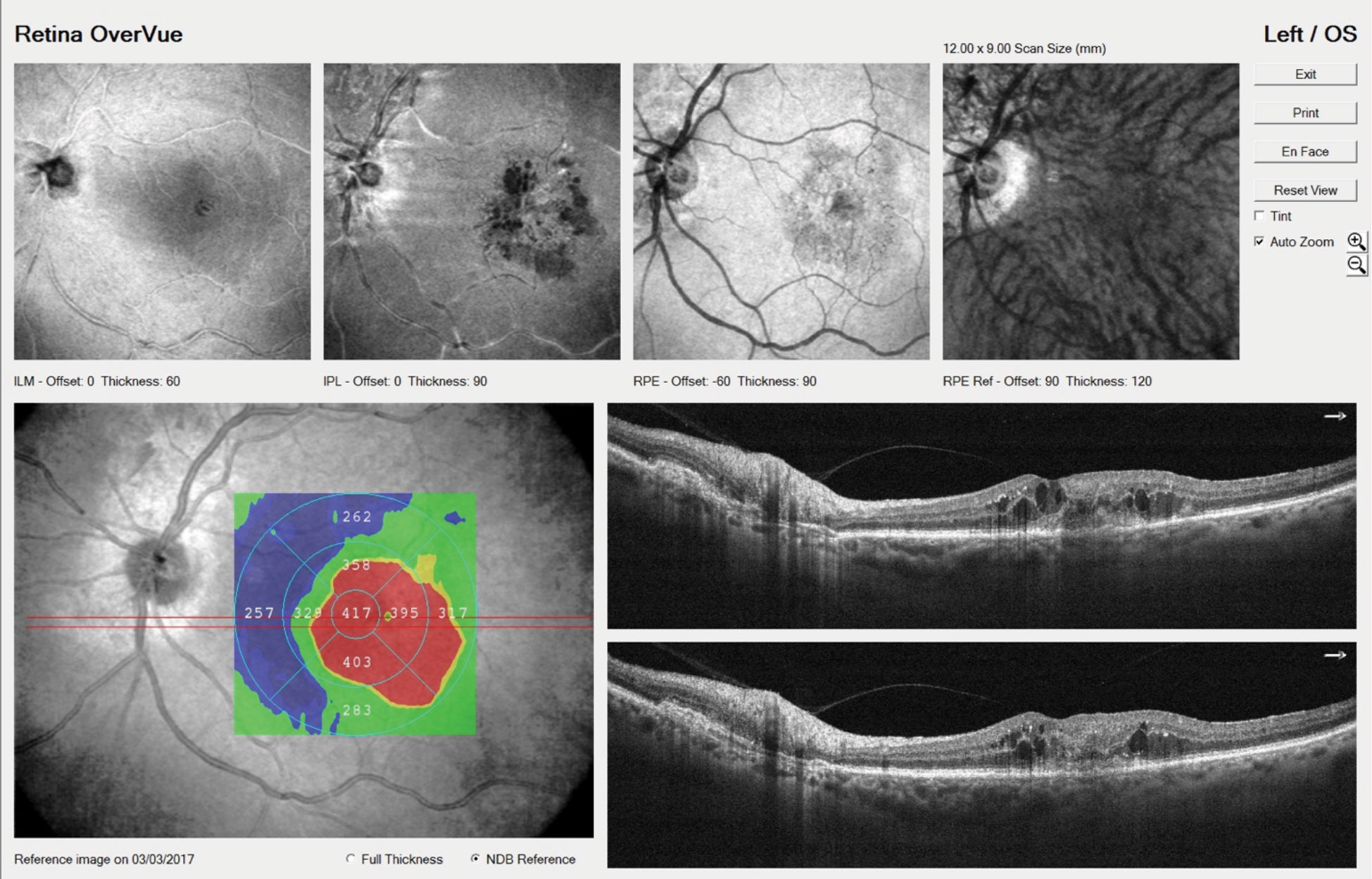 |
| OCT imaging shows the left eye of an 81-year-old CNVM patient with intraretinal fluid. He’ll be treated with intravitreal anti-VEGF injections. Click image to enlarge. |
Intimidating Equipment
Another aspect that might spook patients is the use of a lid speculum. The use of this device is not essential, but patients may be interested to know that some research shows patients for whom a speculum was not used had higher rates of endophthalmitis than those in whom the speculum was used.8 However, that research dates back to 2004—early days in regards to intravitreal injections—so things may have changed.
However, the lids and lashes must be kept away from the needle as they can serve as a potential source of contamination.9 A recent survey of retina specialists revealed that 92% still use a speculum, so chances are patients will have to get comfortable with it.10
Reducing Risks
Cleaning the eye thoroughly, including the lids and lashes, with 5% to 10% povidone-iodine is probably the single best precaution for endophthalmitis, and has emerged as the standard of care for intravitreal injections.11 Research shows that the omission of povidone-iodine is associated with much higher rates of endophthalmitis and, in general, should be the last agent applied to the eye immediately before an injection.11 While povidone-iodine is a necessity, repeated exposure to it does seem to lead to increasing reports of dry eye, which should be managed by the referring optometrist accordingly.12
Any active infection, such as blepharitis, should be treated prior to injection. Eyelid abnormalities, such as ectropion, can increase the risk for endophthalmitis and may need to be addressed as well.13
Also, in the early days of injections, patients were treated with pre- and post-procedural topical antibiotics, typically a fluoroquinolone. However, the literature has not demonstrated that topical prophylactic antibiotics reduce the risk of post-injection endophthalmitis. Further, some researchers believe antibiotic overuse may contribute to antibiotic-resistant bacteria, or have other detrimental effects on ocular surface health.14 For this reason, doctors have mostly abandoned prophylactic antibiotics in these cases.
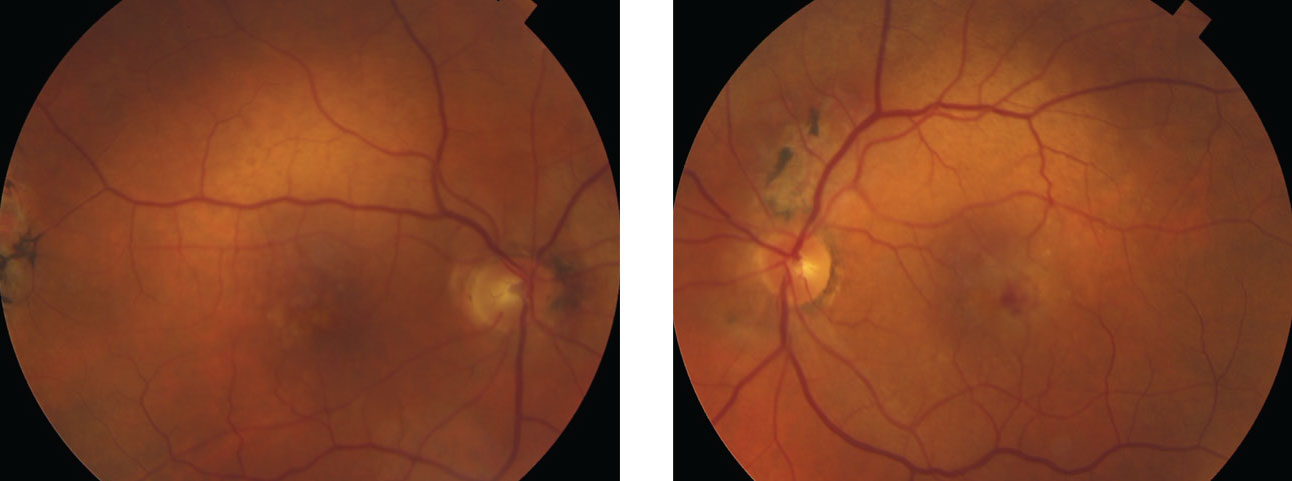 |
| These fundus photos show the same patient from page 50. He had a decreased VA in the left eye. Note the heme still visible in the macula, even after treatment. Anti-VEGF injections helped him maintain best-corrected visual acuities of 20/30 OD, 20/70 OS. Click image to enlarge. |
Drugs in the Literature
Currently, several anti-VEGF agents are available and each have pros and cons. While optometrists don’t make the decision about which agent to inject, a robust understanding of the various agents available may help illuminate the patient’s experience.
Intravitreal anti-VEGF has largely replaced grid or focal lasers for macular edema treatment, both in diabetic retinopathy (DR) and retinal vein occlusions (RVO), and has almost completely replaced the laser treatments for choroidal neovascularization (CNV) of any etiology.
Macugen (pegaptanib, Bausch + Lomb) was the first intravitreal anti-VEGF approved for the treatment of wet age-related macular degeneration (AMD), but has since been supplanted by newer agents.
Lucentis (ranibizumab, Genentech) was first approved for the treatment of wet AMD and has since received approval to treat macular edema following RVO, the treatment of DME, all forms of DR and myopic (CNV). Avastin (bevacizumab, Genentech) is also widely used off label for retinal diseases, but is not FDA approved for the use in the eye. Investigators originally believed the molecule at the heart of it was too large to penetrate the retina, so it was redesigned as Lucentis. However, doctors widely use it off-label, after having a compounding pharmacy reformulate it for intraocular use, in myriad eye diseases, including CNV of any etiology, DME and RVO. Studies conducted worldwide show Avastin injections are comparable to Lucentis in safety and efficacy at less cost.15-17
Eylea (aflibercept, Regeneron) was first FDA approved for wet AMD and has since gained approval for DME and all forms of DR.18-20 In 2018, Eylea received FDA clearance to inject every 12 weeks.18,19 The major advantage, research shows, is that it has longer action than Lucentis, and perhaps requires less frequent injections, decreasing the treatment burden.20
The most recent FDA-approved agent for the treatment of wet AMD is Beovu (brolucizumab-dbll, Novartis). It was FDA approved in October 2019 for the treatment of wet AMD based on the Phase III trials HAWK and HARRIER—which showed non-inferiority to Eylea in best-corrected visual acuity at one year (48 weeks) when injected every three months.21 Approximately 30% of patients gained at least 15 letters by end of one year.21 It also demonstrated greater reduction in central retinal thickness as early as week 16 and at one year vs. Eylea.21 Also, the data showed more than half the patients were maintained on the three months-dosing schedule (56% in HAWK, 51% in HARRIER), with the remaining patients receiving injections every eight weeks.21
However, recent reports have emerged regarding potential adverse effects of Beovu.22 In late February, the American Society of Retina Specialists issued a letter warning its members of 14 cases of vasculitis, 11 of which were reported as retinal arterial occlusive disease.22
Intravitreal steroids, most notably triamcinolone acetate (TA), are also used to treat retinal disorders such as DME, macular edema from RVO and posterior uveitis due to their anti-inflammatory, antiangiogenic and anti-permeability properties. Intravitreal TA was previously widely used for the treatment of DME, until a study showed that laser photocoagulation for DME is more effective over time and has fewer side effects than TA.23 Similar studies with both central and branch RVO show intravitreal triamcinolone are not as effective as anti-VEGF treatment, with more side effects.24
Intravitreal steroids have also been used to treat wet AMD either alone or with anti-VEGF. However, due their side effects, such as cataract formation and increased IOP, intravitreal steroids are generally not first line for the treatment of retinal disease. They are still an option for patients who do not respond to traditional anti-VEGF injections, and as such, are rarely if ever used for AMD.25
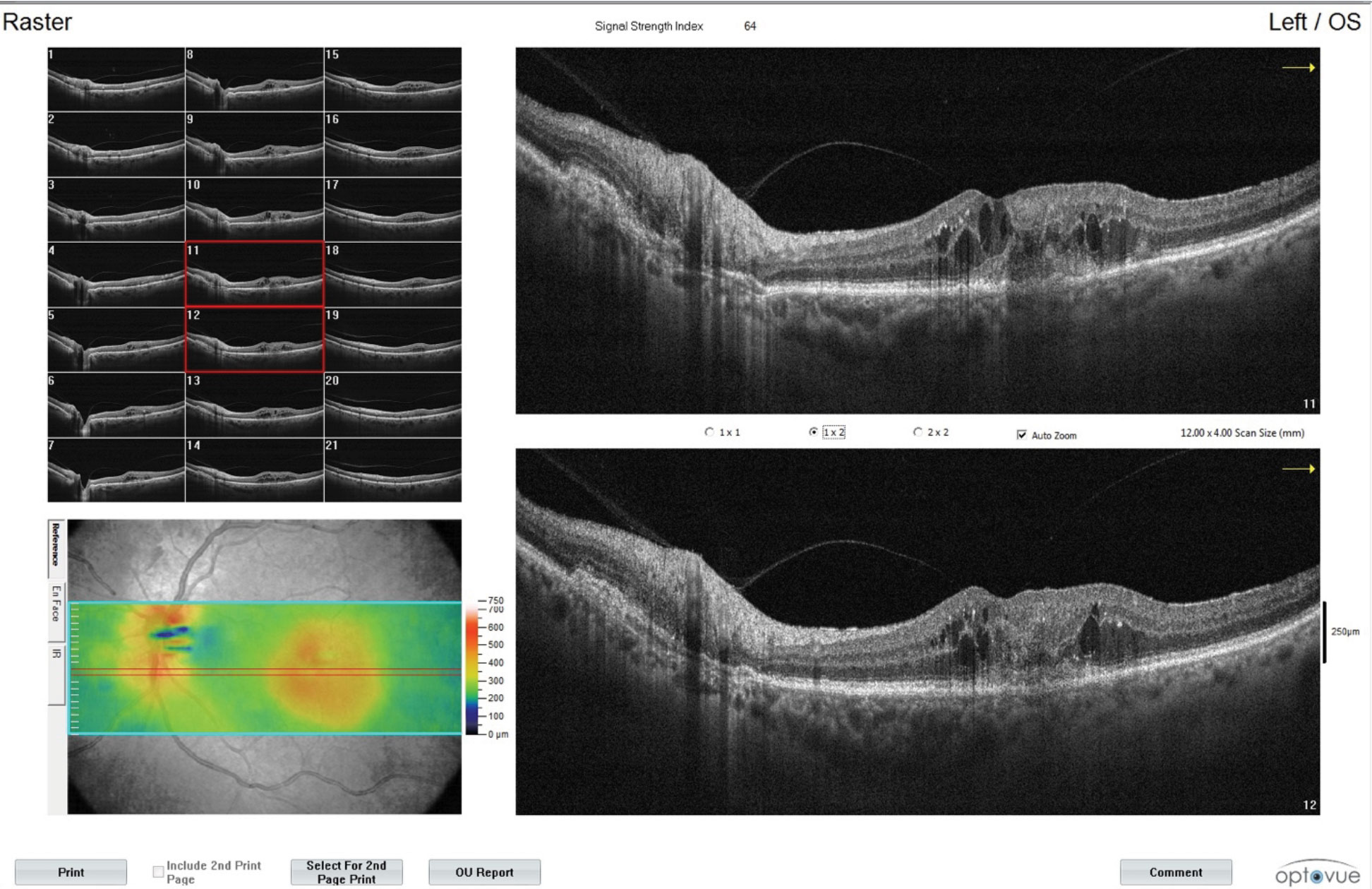 |
| These images show additional findings from the CNVM patient with intraretinal fluid in the left eye. Monitoring is a key part of the OD’s role in managing patients undergoing anti-VEGF shots. Click image to enlarge. |
Monitor IOP
Acute IOP rise after intravitreal injection is common, and typically lasts for a few hours at the most.26 Specialists may check the patient’s IOP after injection, as an acute, severely elevated IOP may lead to central retinal artery occlusion.
Some studies, however, have reported that increased number of injections may be associated with an increased risk for IOP elevation.26 Further, IOP elevation rates are higher in patients with preexisting glaucoma than in those without. While preexisting glaucoma is not a contraindication for intravitreal injection, clinicians must use caution in treating glaucoma patients, and all patients should have their IOP routinely monitored, and treated with either drops or surgery should the IOP become a concern.26
Complication Coaching
The patient should be counseled regarding common complications, such as discomfort, pain and subconjunctival hemorrhage, as well as uncommon but important post-op complications, such as retinal detachment and endophthalmitis. The patient should be advised they may experience slight discomfort for the rest of the day, but significant pain, redness or acute vision loss should warrant a call or office visit.
Reports show subconjunctival hemorrhages occur in nearly 10% of all injections, with higher frequency in patents on aspirin or other blood thinners. Like most subconjunctival hemorrhages, they are self-limiting but can be concerning for the patient, so reassurance is advised.27 However, clinicians should not advise patients to discontinue their blood thinners for the procedure.
Perhaps the most serious complication of intravitreal injections is endophthalmitis. Infectious endophthalmitis following intavitreal injection can be recognized by the presence of pain, redness and a severe anterior reaction consisting of keratic precipitates, hypopyon, fibrin or anterior synechia.28 In clinical trials, the rate of endophthalmitis with anti-VEGF agents was reported to range from 0.019% to 1.6%.29
However, the rate appears lower in more recent studies. In one study, among the 4.3 million injections reviewed, the rate of endophthalmitis was one in 2,771 (0.036%).30 Unlike other studies that seemed to show similar rates among different agents, this study showed aflibercept had a statistically higher rate of suspected endophthalmitis vs. the other agents, at 0.049%.31 A higher rate was found in steroids vs. anti-VEGF agents, with triamcinolone injections having a rate of 0.147%.32 The mean time it took for endophthalmitis to set in was 4.7 days after the injection, with an average visual acuity reduction of 74 letters on presentation.32
Furthermore, approximately 50% of patients who develop endophthalmitis after injection will not return to their pre-injection level of acuity, despite appropriate treatment.32 For this reason, some retina specialists institute a follow-up phone call three to seven days after the injection and inquire about symptoms suggestive of endophthalmitis, such as reduced vision, severe redness or pain.29
Other uncommon side effects include retinal detachments (up to 0.67%) traumatic cataract (<0.1%) and other rare ocular events such as anterior ischemic optic atrophy, RVO, retinal artery occlusions, hemorrhagic macular infarction and sixth nerve palsy.33 Further, corneal abrasions, most likely due to the lid speculum, as well as dry eye from repeated povodine-iodine use, may also be infrequently encountered.34
No clinical evidence shows what patients should avoid after injections, but in theory, attempts to limit the exposure of microorganisms near the site of injection is practical. Many physicians tell patients to avoid swimming, rubbing eyes, gardening, wearing makeup or performing dusty work for 24 hours.34
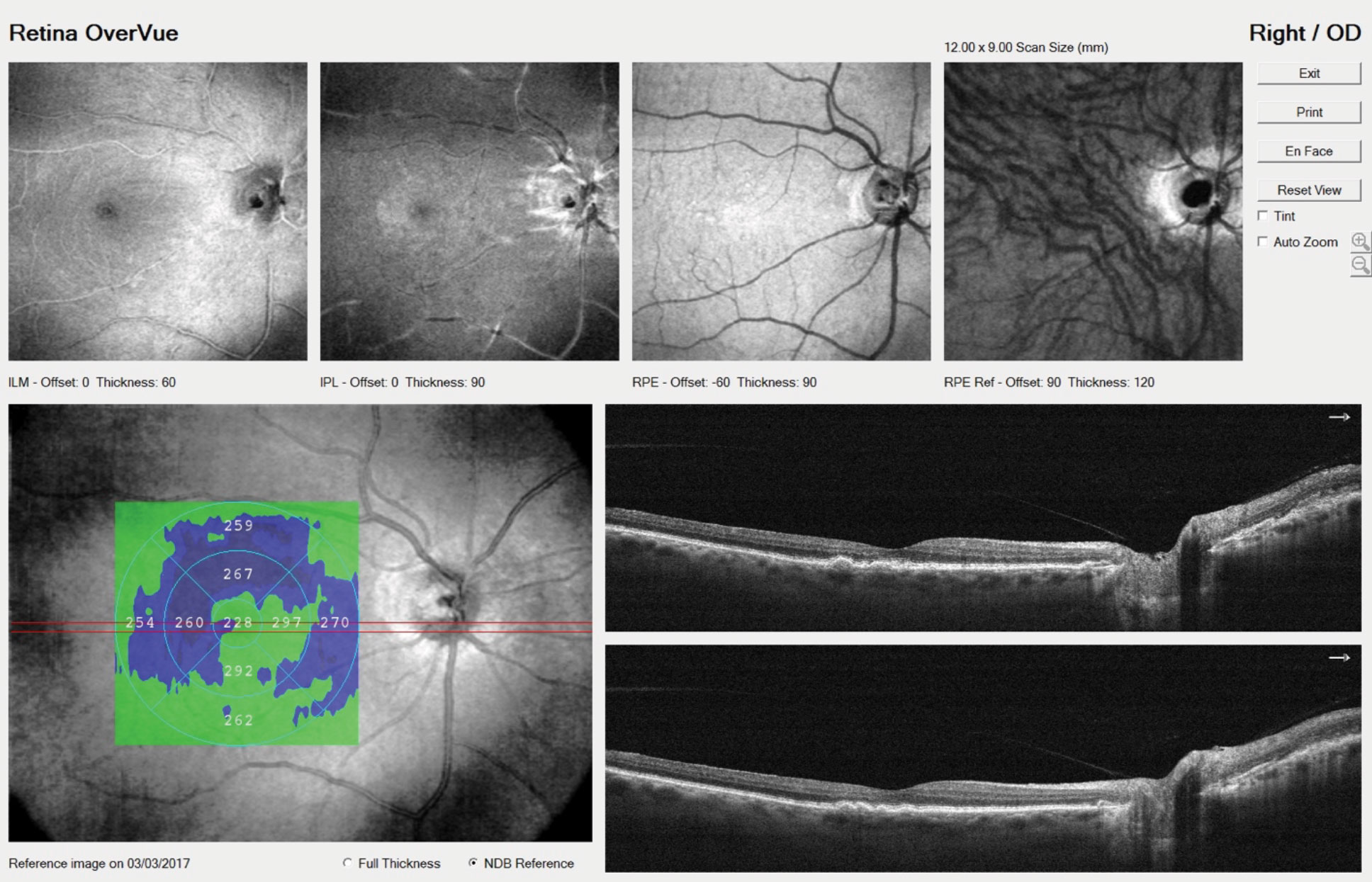 |
| OCT identified a few small drusen in our patient’s right eye. Increasingly, patients who undergo anti-VEGF injections are now being treated bilaterally to reduce the logistical elements of the treatment burden. Click image to enlarge. |
On the Horizon
Treatment with intravitreal anti-VEGF agents is not curative for any disease. Many patients require long-term therapy, with some requiring monthly injections. This presents a high burden of treatment to patients, caregivers and physicians. Patients may become discouraged by their need for ongoing treatment. There is significant effort being put into the development of medications that work better and last longer, in addition to delivery systems that would allow less frequent dosing. While the following treatment options are still in clinical trials, this type of information may be encouraging to share with patients who are dismayed by the idea of needing regular injections long-term.
One of these promising new agents, faricimab (Genentech/Roche), is the first bispecific antibody designed for intravitreal use.35,36 Essentially, it is one molecule with two agents on two separate arms; one arm binds to and inactivates VEGF, similar to the current anti-VEGF agents.35,36 The second arm binds to and inactivates angiopoietin-2.36 Research shows angiopoietin-2 levels are elevated in retinal vascular diseases, such as wet AMD, DR and vein occlusions.35,36 It also appears to play a role in angiogenesis, vascular instability and inflammation in the retina, which makes it an attractive target for treatment.35,36
The Phase II BOULEVARD study evaluated patients with DME treated with faricimab vs. Lucentis.35 The study showed that faricimab had gains in best-corrected visual acuity at six months, with a mean of 13.9 letters gained from baseline.35 The decrease in central retinal thickness was actually better than Lucentis, with no safety concerns.35
YOSEMITE and RHINE Phase III studies are currently underway comparing faricimab with Eylea for DME, and represent the largest DME studies to date.37,38
The AVENUE and STAIRWAY Phase II studies evaluated faricimab in wet AMD.39 In the studies, patients were treated with faricimab either every four weeks or every eight weeks and compared with Lucentis every four weeks. The STAIRWAY study evaluated faricimab every 16 or 12 weeks.39 The results show that 65% of faricimab patients had no disease activity at 12 weeks and visual acuity was comparable to that of Lucentis every four weeks. Further central retinal thickness was reduced and a decrease in size of CNVM lesions was noted.39 Phase III studies comparing faricimab every 16 weeks to Eylea every eight are currently underway.40,41
Other research is looking at alternative methods. One of these, the Port Delivery System (Genentech/Roche) with ranibizumab is a reusable, surgically implanted drug reservoir placed through a scleral incision in the pars plana.42-44 It can hold approximately 20 microliters of ranibizumab (of a slightly different formulation than Lucentis), which it diffuses continuously into the vitreous.42-44 When empty, the device can be refilled in-office using a specialized refill needle, eliminating the need for monthly intravitreal injections.42-44 The Phase II LADDER study evaluated more than 200 patients with exudative AMD with three different concentrations of ranibizumab in the reservoir vs. monthly injections of Lucentis.42,43 Overall, the vision was similar across all treatment arms, with an average best-corrected vision of 20/40.42,43 The procedure was relatively safe, with few cases of endophthalmitis and retinal detachment.42-44 The refill procedure, done under local anesthesia, is also well tolerated.42-44
By developing a better understanding of the procedure of intravitreal injections—and providing proper comanagement of concomitant disease such as dry eye, blepharitis and glaucoma—the primary care optometrist can better counsel their patient on what to expect when referred to a retina specialist for treatment. New advancements in injections, such as longer acting agents and alternate delivery systems, may hold hope for optometric patients as well as the profession’s future.
Drs. Ferrucci and Yeh practice at the Department of Veterans Affairs in North Hills, Calif., and are on faculty at the Southern California College of Optometry at Marshall B. Ketchum University in Los Angeles.
| 1. Singer M, Dhoot D. Retina minute: review of intravitreal injections and endophthalmitis. Ret Phys. retinalphysician.com/newsletter/retina-minute/february-2020. February 2020. Accessed May 25, 2020. 2. Saddine J, Honeycutt A, Narayan K, et al. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005-2050. Arch Ophthalmol. 2008;126:1740-7. 3. Cheung C, Wong A, Lui A et al. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injection. Ophthalmol. 2012;119:1609-14. 4. McCannel C. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31:654-61. 5. Pilli S, Kotsolisa A, Spaide R, et al. Endophthalmitis associated with intravitreal anti-vascular endothelia growth factor therapy injection in an office setting. Am J Ophthalmol. 2008;145:879-82. 6. Giocanti-Aurgean A, Tadayoni R, Grenet T, et al, Estimation of the need for bilateral intravitreal anti-VEGF injections in clinical practice. BMC Ophtlmol. 2016;16(1):142. 7. Shiroma HF, Takaschima A, Farah M, et al. Patient pain during intravitreal injections under topical anesthesia: as systematic review. Int J Retina Vitreous. 2017;3(7):23. 8. Gragoudas E, Adamis A, Cunningham E, et al. Pegatanib for neovascular age-related macular degeneration. N Engl J Med. 2004:351(27):2805-16. 9. Fineman M, Hsu J, Spirn M, Kaiser R. Bimanual assisted lid retraction technique for intravitreal injections. Retina. 2013;33(9):1968-70. 10. Green-Simms AE, Ekdawi N, Bakriet S, et al. Survey of intravitreal injection techniques among retinal specialists in the United States. Am J Ophthalmol. 2011;151(2):329-32. 11. Bhasavar A, Glassman A, Stockdale C, et al. Elimination of topical antibiotics for intravitreous injections and the importance of using povidone iodine: update from the diabetic retinopathy clinical research network. JAMA Ophthalmol. 2016;134(10):1181-3. 12. Saedon H, Nosek J, Phillips J. Ocular surface effects of repeated application of povisoden-iodine in patients receiving frequent intravitreal injections. Cutaneous and Ocular Toxicology. 2017;36(4):343-6. 13. Speaker M, Milch F, Shah M, et al. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmol. 1991;98:639-50. 14. Storey P, Dollin M, Pitcher J. The role of topical antibiotic prophylaxis to prevent endophthalmitis after intravitreal injection. Ophthalmol. 2014;12(1):283-9. 15. The CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897-908. 16. IVAN Study Investigators. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: One year findings from the IVAN randomized trial . Ophthalmol. 2012;119(7):1399-411. 17. Kodjjkian L, Souied E, Mimoun G, et al. Ranibizumab versus Bevacizumab for Neovascular Age-Related Macular Degeneration: Results from the GEFAL Noninferiority Randomized Trial. Ophthalmology 2013;120(110):2300-9. 18. Clinicaltrials.gov. Study report: VGFT-OD-0605 (Year 1): a randomized, double masked, active controlled phase 3 study of the efficacy, safety, and tolerability of repeated doses of intravitreal VEGF trap in subjects with neovascular age-related macular degeneration VEGF trap-eye: investigation of efficacy and safety in wet AMD (VIEW 1). clinicaltrials.gov/ct2/show/NCT00637377. January 25, 2011. Accessed May 25, 2020. 19. Schmidt-Erfurth U, Chong V, Kirchof B, et al. Primary results of an international phase III study using intravitreal VEGF trap-eye compared to ranibizumab in patients with wet AMD (VIEW 2). Invest Ophthalmol Vis Sci. 2011;52:E-Abstract 1650. 20. Heier JS, Brown D, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmol. 2012;119(12):2537-48. 21. Dugel P, Koh A, Ogura Y. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmol. 2020;127(1):72-84. 22. What Novartis hopes to learn from its review of Beovu AEs. Retina Specialist. 2020;6(2):6. 23. DRCR Network. Three-year follow-up of a randomized trail comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127(43):245-51. 24. Jonas J, Paques M, Monés J, Glacet-Bernard A. Retinal vein occlusions Dev Ophthalmol. 2010;47(8):111-35. 25. Sarao V, Veritti D, Boscia F, Lanzetta P. Intravitreal steroids for the treatment of retinal diseases. ScientificWorldJournal. 2014:8(1):1-14. 26. Good TJ, Kimura A, Mandava N, Kahook M. Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. Br J Ophthalmol. 2011;95:1111-4. 27. Ladas ID, Karagiannis D, Rouvas A, et al. Safety of repeat intravitreal injections of bevacizumab versus ranibizumab: our experience after 2,000 injections. Retina. 2009;29(3):313-8. 28. Mezad’Koursh D, Goldstein M, Heilwail G, et al. Clinical characteristics of endophthalmitis after an injections of intravitreal antivascular endothelial growth factor. Retina. 2010;30:1051-57. 29. McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Retina 2011;31:654-661. 30. Xu K, Chin E, Bennett S, et al. Endophthalmitis after intravitreal injection of vascular endothelial growth factor inhibitors: management and visual outcome. Ophthalmol. 2018;125(8):1279-86. 31. Falavarjani K, Nguyen Q. Adverse events and complications associated with intravitreal injection of ant-VEGF agents; a review of the literature. Eye 2013;27(7):787-94. 32. Petri A, Boysen K, Cehofski L, et al. Intravitreal injections with vascular endothelial growth factor inhibitors: a practical approach. Ophthalmol Ther. 2020;9(1):191-203. 33. Regula J, von Leithner PL, Foxton R, et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med. 2016;8(11):1265-88. 34. Frenkel RP, Haji S, La M, et al. A protocol for the retina surgeon’s safe initial intravitreal injections. Clin Ophthalmol. 2010;4:1279–85. 35. Khanani AM. Anti-VEGF/anti-angiopoietin-2 bispecific antibody RG7716 in diabetic macular edema: results from the phase 2 BOULEVARD clinical trial. Paper presented at the World Ophthalmology Congress, Barcelona, Spain, June 18, 2018. 36. Khanani AM. Simultaneous inhibition of VEGF and Ang2 with faricimab in neovascular AMD: STAIRWAY phase 2 results. Paper presented at the Retina Subspecialty Day, American Academy of Ophthalmology Meeting, Chicago, Illinois, October 26, 2018. 37. Clintrials.gov A Study to Evaluate the Efficacy and Safety of Faricimab (RO6867461) in Participants With Diabetic Macular Edema (YOSEMITE). clinicaltrials.gov/ct2/show/NCT03622580?term=yosemite&rank=1. April 21, 2020. Accessed May 25, 2020. 38. Clintrials.gov. A Study to Evaluate the Efficacy and Safety of Faricimab (RO6867461) in Participants With Diabetic Macular Edema (RHINE). clinicaltrials.gov/ct2/show/NCT03622593?term=Rhine&rank=2. April 21, 2020. Accessed May 25, 2020. 39. Kirkner R. What’s next for bispecific antibody faricimab. Retina Specialist. www.retina-specialist.com/article/whats-next-for-bispecific-antibody-faricimab. November 11, 2018. Accessed May 25, 2020. 40. Clintrials.gov. A study to evaluate the efficacy and safety of faricimab in participants with neovascular age-related macular degeneration (TENAYA). clinicaltrials.gov/ct2/show/NCT03823287. May 27, 2020. Accessed May 27, 2020. 41. Clintrials.gov. A study to evaluate the efficacy and safety of faricimab in participants with neovascular age-related macular degeneration (LUCERNE). clinicaltrials.gov/ct2/show/NCT03823300. April 21, 2020 Accessed May 27, 2020. 42. Awh C. LADDER trial of the Port Delivery System for ranibizumab: preliminary study results. Presented at the 36th Annual Meeting of the American Society of Retina Specialists; Vancouver, British Columbia, Canada; July 25, 2018. 43. Pieramici D. LADDER trial of the Port Delivery System with ranibizumab: initial study results. Presented at the Retina Society 2018 annual meeting; San Francisco, California; September 14, 2018. 44. Pieramici D. Port Delivery System with ranibizumab (PDS): from dose ranging in LADDER phase 2 to ARCHWAY phase 3 study design. Presented at the 2018 American Academy of Ophthalmology Annual Meeting; Chicago, Illinois; 2018 Oct 27. |

