 |
A 76-year-old white female presented in late April for optical coherence tomography (OCT) and Heidelberg retinal tomography (HRT 3) images of her optic nerves, related to her long-standing glaucoma.
Her previous visit was five months earlier, at which time visual fields, intraocular pressure (IOP) readings and a slit lamp evaluation of her optic nerves were all stable.
History
Her medications included aspirin, Lipitor (atorvastatin, Pfizer), Lopressor (metoprolol, Novartis), Paxil (paroxetine, GSK), K+ supplementation, Nasonex (mometasone, Merck) and Claritin (loratadine, Bayer). She has allergies to multiple medications, including all penicillins and several antihypercholesterolemia medications. She has an extensive history of cardiovascular disease with coronary artery bypass grafting x4 and subsequent coronary artery stenting x7 over the past 10 years.
Her most recent cardiovascular event, in 2015, required surgical intervention. Three months prior to that, she underwent an appendectomy and recovered well.
She presented as a new patient in 2006, carrying with her a diagnosis of open-angle glaucoma, at which time she was medicated with Xalatan (latanaprost, Pfizer) HS OU and 0.5% timolol BID OU. Her central corneal thicknesses were 517µm OD and 514µm OS. In the first few visits in late 2006 and early 2007, she was deemed to be relatively stable insofar as her glaucoma was concerned, and her glaucoma state was graded as severe at that time, with optic nerves appearing similarly to the current presentation.
Her current glaucoma medications include Azopt (brinzolamide, Novartis) BID OU and Lumigan (bimatoprost, Allergan) HS OU. Best-corrected visual acuities were 20/20 OD and 20/25- OS through hyperopic astigmatic and presbyopic correction. She was pseudophakic and underwent cataract surgery in 2009.
 |
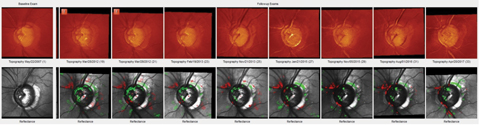
| The most recent images of this patient’s left eye demonstrate significant cupping and a markedly reduced ganglion cell layer overlying Bruch’s membrane opening, consistent with advanced glaucomatous disease. Click image to enlarge. |
Testing
The slit lamp exam of her anterior segments was essentially unremarkable. There was mild corneal arcus noted in both eyes, along with minimal endothelial cell loss. The anterior chamber angles were open, and the chambers were deep and quiet with no cells or flare. Applanation tensions were 13mm Hg OD and 14mm Hg OS, consistent with previous visits. Pupils were equal, round, responsive to light and accommodation with no afferent pupil defect. Her intraocular lenses were in their capsular bags and both posterior capsules had been opened following implantation. Through dilated pupils, she had long-standing bilateral peripheral vascular diseases. Her cup-to-disc ratios were 0.75 x 0.85 OD and 0.85 x 0.95 OS, which were consistent with previous evaluations.
The remaining neuroretinal rims were plush and well perfused, but there was significant thinning of the rim temporally, moreso in the left eye than the right. Both maculae were characterized by fine RPE mottling and a few drusen, with no evidence of subretinal neovascular membrane in both eyes.
The patient’s retinal vasculature was characterized by grade 2 arteriolarsclerotic retinopathy and minimal hypertensive retinopathy in both eyes. Her peripheral retinal evaluations were unremarkable.
Scans
HRT 3 and OCT imaging of both optic nerves was obtained, with good image quality. Both the HRT 3 scans, as well as the OCTs, demonstrated no progression of the glaucomatous damage in the neuroretinal rims, the perioptic retinal nerve fiber layer and the macular ganglion cell layers.
She has remained stable for 10 years—save for the cataracts, and a gradual creep in her IOP which required bilateral SLTs and, ultimately, a change in her topical glaucoma medications.
 |
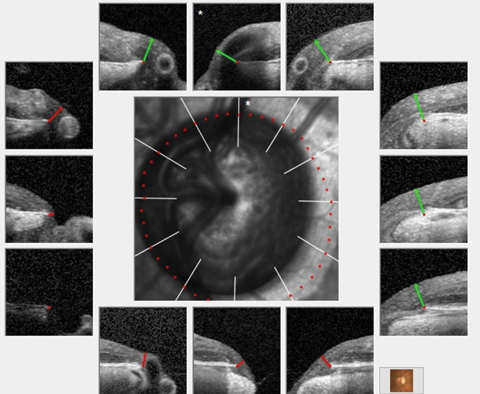
| The patient’s HRT 3 scans of her left eye over the past 10 years showing marked stability of her neuroretinal rims in the presence of advanced glaucoma. Click image to enlarge. |
Discussion
This long-term patient initially presented with advanced glaucoma, and, over the past 10 years, developed expected changes, such as gradual worsening.
When establishing care with new patients, it’s important to evaluate their overall stability within the first couple of visits and begin to develop a doctor/patient relationship. Of course, obtaining old records at that time is helpful in determining stability, but as you manage these patients over the course of their lives, you will ultimately be repeating all of those same studies. The data gathered over decades of care becomes the patient’s own reference data base, upon which future stability is continually evaluated. IOP ranges, structural details of the optic nerve (as obtained by CLSO, OCT and stereo photos), functional details of the optic nerve (as obtained by threshold visual fields), compliance issues (if any), medication tolerability and ancillary ophthalmic findings are all elucidated over time.
Given a relatively compliant patient, it becomes easy to see trends over time. We know that ultimately, glaucoma patients are going to follow one of two paths: they will either remain stable or get worse. Our primary responsibility to the patient is to determine whether they are indeed stable. If so, then maintaining the status quo is prudent. If not, a course change is required. As captain of that ship, determining when to change course and in what direction to change course is critical in allowing the patient to continue seeing well enough to live out the rest of their lives. Clinicians who manage glaucoma must be comfortable in being the “captain” of that ship. Certainly, there will be instances where consultation will be necessary but ultimately if you are planning on managing glaucoma, you need to understand, accept and be comfortable with managing glaucoma patients of all different types—even those with advanced disease.
How you handle the doctor-patient relationship can either soothe or discomfort the patient. Our patient was a new patient with advanced disease who has chosen me to be her provider of glaucoma care. Eleven years have lapsed since we first met. She has undergone selective laser trabeculoplasty and modification of therapy because of some instability in her disease, yet we were able to wrest control of the situation. Of course, the other way that this might go is that she progresses, and loses vision.
With advanced glaucoma patients, the unfortunate reality is they may lose their vision, but with the right kind of care, the optometrist can be the hand the patient holds across that threshold.

