Take Charge of GlaucomaFollow the links below to read other articles from our four-part glaucoma series: |
Glaucoma is the leading cause of irreversible blindness in the world.1 And, while researchers are still speculating about the precise etiology and mechanism of the disease and the damage it causes to the optic nerve, one modifiable risk factor is known—intraocular pressure (IOP). Ocular hypotensive topical medications, laser procedures and numerous surgeries all aim to lower IOP and, ideally, stem any new damage.
While all optometrists can diagnose glaucoma and most have the legal privilege to treat glaucoma with drops, the number of ODs who participate in postoperative care of glaucoma patients is still growing. Within the last five years alone, we have seen an explosion of new surgical procedures available. This article reviews glaucoma-related procedures along with the postoperative care and the vital role optometrists can embrace.
Laser Peripheral Iridotomy
This procedure creates a hole in the iris that ideally causes an equalization of pressure between the anterior and posterior chambers. The laser peripheral iridotomy (LPI) procedure is performed with either an Nd:YAG laser, argon laser or a combination of the two. At one point, LPIs were done superiorly.2 A 2014 study challenged that idea and many clinicians moved the placement to the temporal iris to reduce the chance of linear dysphotopsia.2 However, a recent study is challenging that idea by concluding that linear dysphotopsia is independent of LPI location.3
One of the most underused techniques in our diagnostic toolkit is angle assessment. This is typically achieved by gonioscopy. Angle imaging such as angle OCT or B-scan is complementary to gonioscopy and becoming more common. All referrals should include angle assessment. Take care not to erroneously label chronic narrow angle glaucoma cases as primary open angle glaucoma (POAG) cases. Always assess the angles prior to any type of laser or surgical intervention, whether you’re comanaging with an ophthalmologist or you’re in a state where optometrists can perform an LPI themselves.
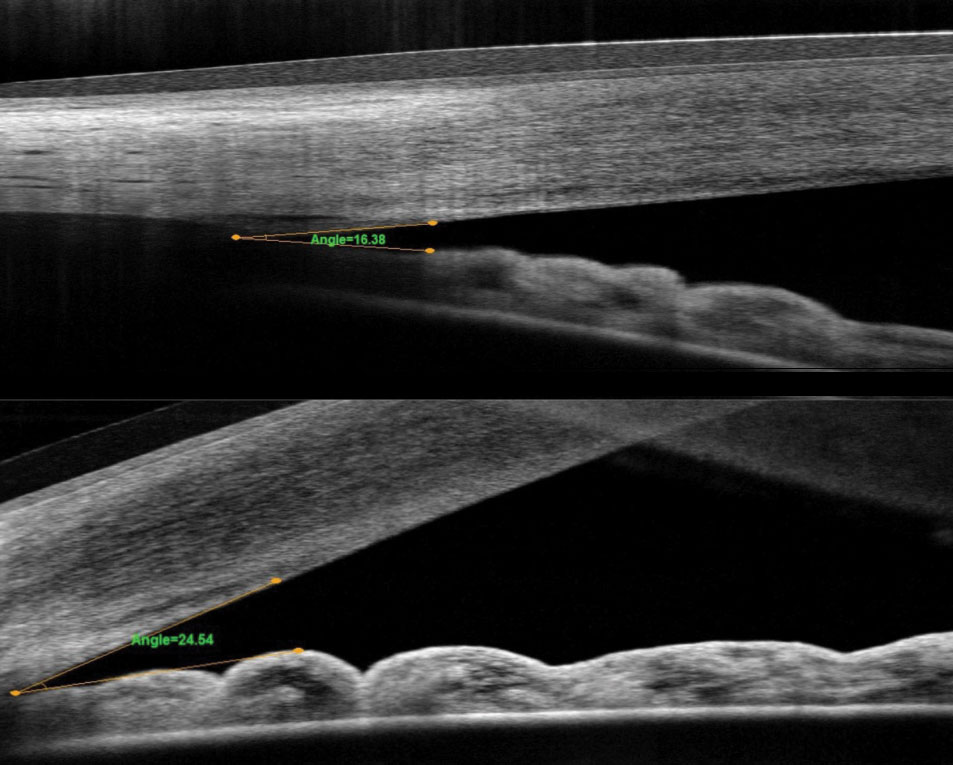 |
| Fig. 1. These OCT images compare the angle pre-(top) and post-LPI (bottom). |
Narrow angle is a difficult concept for most patients to understand. Convincing an asymptomatic patient to have a laser procedure can be challenging. One helpful tool is to show patients their actual angle. In our practice, we show them the OCT angle pre- and post-LPI (Figure 1). In the patients whose angles open with LPI, we explain that this is a temporary fix and that the angle will continue to narrow as the natural lens becomes thicker. We help them understand that cataract surgery may eventually be needed to open the angle.
Should we consider clear lens extraction as first-line treatment for the treatment of primary angle closure or primary angle closure glaucoma if the patient did not have a cataract? At one time, the standard of care was LPI followed only by clear lens extraction if the angle remained closed. The EAGLE study challenges that idea, saying “clear lens extraction showed greater efficacy and was more cost-effective than laser peripheral iridotomy, and should be considered as first line treatment.”4
The postoperative LPI patient typically uses 1% prednisolone acetate for four days. The optometrist should evaluate the patient approximately two weeks afterwards to assess the anterior chamber, IOP and angle via gonioscopy or angle imaging, or both. Complications can include a temporary rise in IOP, hyphema and inflammation.
 |
| Click image to enlarge. |
SLT Procedure
This laser targets the pigmented cells of the trabecular meshwork (TM) to promote aqueous outflow. Using it can also reduce the IOP by approximately 25% after three years.5
Research shows the use of topical glaucoma drops can reduce a patient’s quality of life.6 Eye drop compliance may also hinder therapeutic IOP reduction.7 There is currently no way of knowing whether a patient is taking their drops properly. Selective laser trabeculoplasty (SLT) is designed to reduce that medication burden while maintaining adequate IOP reduction.8 SLT may also be considered a first-line therapy.9
The postoperative SLT patient may be on topical steroids, topical NSAIDs or nothing, as the literature is inconclusive on which option is the most effective. In our practice, patients typically are on no drops afterwards. Early results from the SALT (Steroids After Laser Trabeculoplasty) trial show a short-term IOP-lowering effect with topical NSAIDs compared with placebo.10 We’re currently evaluating whether to modify our postoperative SLT regimen.
The patient should be evaluated approximately one to two weeks after SLT to monitor IOP. Spikes may occur more frequently in pigmentary glaucoma and exfoliation glaucoma.11,12 The anterior chamber should also be carefully inspected for any sign of inflammation. As SLT may take two to four months to reach peak effect, the next visit should fall around the two to four month time-frame. Success rates of SLT are between 75% to 97% at six months to a year.9,13,14
Other side effects of SLT include conjunctival injection, discomfort, headache, photophobia, corneal abrasion and subconjunctival hemorrhage.
 |
| Fig. 2. This photo shows a patient’s angle following the Kahook Dual Blade (KDB) procedure. Note the white KDB cleft. |
Minimally Invasive Glaucoma Surgeries
This class of procedures is an option for mild-to-moderate glaucoma patients that both lowers the medication burden, as most can reduce the number of drops they are taking, and lowers IOP.16 A large meta-analysis shows minimally invasive glaucoma surgeries (MIGS) have a good safety profile, with IOP spikes being the most frequent complication.16 Clinicians who perform postoperative care for MIGS patients should be aware that as many as 90% of POAG patients will have an ocular hypertensive response to topical steroids.17 It is important for the clinician to be vigilant about IOP spikes and be prepared to mitigate steroid responders as early as the one-week postoperative visit. Many of these procedures involve cataract surgery, and glaucoma is a strong risk factor for developing elevated IOP after cataract surgery.18
MIGS procedures include:
1. Internally-applied laser (e.g., endocyclophotocoagulation).
2. Trabecular surgery (e.g., Kahook Dual-Blade, Trabectome, iStent).
3. Suprachoroidal shunts (e.g., Cypass).
4. Microtrabeculectomies (e.g., Xen).
Endocyclophotocoagulation (ECP) (Beaver-Visitec International). This procedure employs a microprobe laser to coagulate necrotic damage to the ciliary body epithelium, while sparing the ciliary muscle and vasculature. Ciliary body damage reduces aqueous flow.19 ECP can be combined with phacoemulsification or can be performed in aphakia. The ECP laser is typically performed with 360 degrees of treatment. ECP reduces the IOP around 10% when combined with cataract surgery.20
The postoperative care is similar to standard cataract surgery with a topical antibiotic used for one week and a topical steroid and NSAID used for one week while tapering out to four weeks.
Postoperative complications can include IOP spikes, hyphema and uveitis. IOP spikes are common as early as the one-day postoperative visit. Uveitis may resolve quickly but may sometimes be persistent. Care must be taken when managing a longer-lasting uveitis as prolonged steroid use may trigger a steroid response. The complication rate is similar between cataract surgery and ECP vs. cataract surgery alone.21
ECP is one of the few glaucoma procedures that can be repeated and titrated without leaving any conjunctival scars, nor does it alter the outflow pathway anatomy in any way that would preclude any future glaucoma surgical options.
The iStent (Glaukos) is a non-magnetic titanium shunt implanted into Schlemm’s canal to bypass the TM, in an effort to increase aqueous drainage. Cataract surgery combined with iStent reduces IOP around 10% and can reduce the need for topical medications.22 The FDA has approved the use of one stent, but recent studies show that applying two implants to the same eye may be superior.23,24 Currently, the iStent is always combined with cataract surgery and the postoperative care is the same as cataract surgery alone. Potential complications are similar to cataract surgery too and include corneal edema, anterior chamber inflammation, IOP spikes, hyphema and macular edema.25
The Kahook Dual-Blade (KDB) (New World Medical) device removes a portion of the trabecular meshwork and exposes Schlemm’s canal. The surgically created cleft increases aqueous outflow which leads to more sustained IOP control (Figure 2). KDB combined with cataract surgery reduces the IOP around 24%.26
The KDB can be performed with or without cataract surgery. The postoperative care is the same as cataract surgery alone with a topical antibiotic applied for one week and a topical steroid and NSAID used for one week while tapering out to four weeks.
Postoperative complications may include hyphema on the first postoperative day and IOP spikes which typically occur beginning at the one week visit and may persist until discontinuation of the topical steroid. In our clinic, we apply 1% pilocarpine a few minutes after the steroid drop and follow the same steroid taper. This helps to blunt the IOP spikes.
 |
Fig. 3. The Xen gel stent regulates the flow of aqueous from the anterior chamber into a bleb in the subconjunctival space. |
Trabectome is an energy-delivering electrode probe that causes plasma-mediated TM ablation increasing aqueous outflow. Trabectome can be used with or without cataract surgery. Trabectome combined with cataract surgery reduces IOP around 30% and may be higher in exfoliation patients.27
The postoperative care is the same as cataract surgery alone. As with KDB, pilocarpine can be used to minimize postoperative IOP spikes.
Postoperative complications include hyphema, IOP spikes and, rarely, cyclodialysis.28
The Cypass Micro-Stent (Alcon) is a 6mm long, biocompatible, fenestrated tube implant that creates an alternate outflow chamber into the supracilliary and suprachoroidal space increasing aqueous outflow. Cypass combined with cataract surgery reduces IOP by around 30%.29
Currently, the Cypass Micro-Stent is always combined with cataract surgery and the postoperative care is the same as cataract surgery alone. A topical antibiotic applied for one week and a topical steroid and NSAID used for one week while tapering out to four weeks.
Postoperative complications are similar to cataract surgery alone and include hyphema, IOP spikes, iritis, myopic shifts and corneal edema. In the COMPASS trial, 2.9% developed hypotony but all resolved.29
The Xen gel stent (Allergan) is 6mm-long, consisting of gelatin cross-linked with glutaraldehyde. The implant is stiff when dehydrated but becomes flexible and conforms to the appropriate space when hydrated by the aqueous humor.30 The gel stent regulates the flow of aqueous from the anterior chamber into a bleb in the subconjunctival space (Figure 3). The Xen procedure combined with cataract surgery reduces the IOP around 30%.31
The bleb is typically lower and more diffuse than with a trabeculectomy. The Xen procedure may be performed with or without cataract surgery.
Post-op complications include hyphema, malposition, conjunctival perforation, hypotony and erosion. One drawback of the Xen is that a high percentage of patients may require a needling procedure.
Comanagement with CareFor much of my optometric career, I served on the front lines of glaucoma care and made referrals to glaucoma specialists when surgical intervention was appropriate. Two years ago, our practice partnered with a glaucoma surgeon. Part of my practice is now a referral-based glaucoma service. Seeing referrals from other eye care physicians has allowed me to better appreciate the importance of effective comanagement. One of the best ways to improve your relationship with a glaucoma referral center or glaucoma specialist is to improve the quality of your referral letter. One publication assessed that the quality of glaucoma referral letters found 34% of referral letters substandard.1The five most important pieces of information omitted from the letters were: 1. Serial visual fields. When sharing serial disc imaging, black and white faxes or copies of OCTs are difficult to read and interpret. Sending color OCT printouts will improve patient care. Ask your glaucoma specialist how they wish to be contacted when an urgent issue arises. Is the IOP higher than expected? Is the anterior chamber reaction greater than expected? Is the acuity reduced more than expected? Some may prefer you call the main office number, and others may prefer you call, or text, their cellphones directly. Communicating a potential problem early in the process may prevent a small issue from becoming a large one. Non-urgent communication is also important. Why does that iStent placement look different than usual? Why was a goniotomy performed when it seemed as though an SLT would suffice? Find out how your glaucoma surgeon wishes to be contacted to discuss these items. Some may prefer emails while others prefer phone calls or even periodic lunches. Conversely, a glaucoma specialist’s letter should clearly show their findings, management plan and precisely when the patient will be returned to your care. There should be no ambiguity. Let the specialist know if their letters are not providing you with the information needed to effectively manage the postoperative care. Communication with your glaucoma surgeon is always a two-way street. Effective communication between the referring optometrist and the glaucoma surgeon leads to a trusted relationship and exceptional patient care.
|
Trabeculectomy
This involves bypassing the resistance of the trabecular meshwork and Schlemm’s canal by moving aqueous directly to the subconjunctival space, creating a filtering bleb. This is very effective at reducing IOP but carries a higher risk of complications. Trabeculectomy will reduce IOP from approximately 30% to 50%.33 This procedure is commonly used with the antimetabolite mitomycin C.
After a trabeculectomy, patients should be seen at one day and then every two to three days for approximately two weeks. The patient can then be followed every one to two weeks until two to three months after the procedure. The topical steroid needs to be adjusted throughout this process depending on inflammation and IOP.
Postoperative complications include hypotony, flat anterior chamber, choroidal effusion, choroidal hemorrhage, maculopathy, endophthalmitis and blebitis.34 Inadequate tension in the scleral sutures may be responsible for many of the short-term complications such as hypotony and a releasable suture technique may result in better outcomes.35 A longer-term complication is early cataract development. The overall failure rate is close to 50% at five years post-surgery.
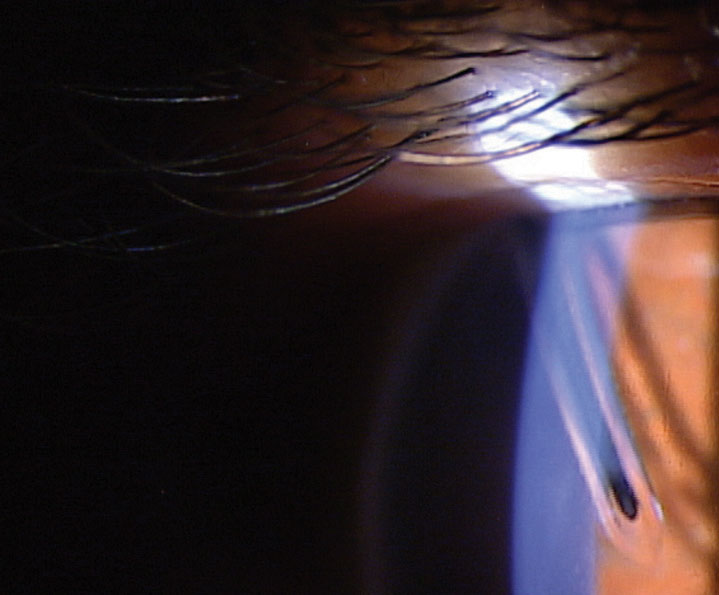 |
| Fig. 4. Baerveldt tube with rip-cord suture still in place. Note the tube’s proximity to the endothelium. |
Tubes
Tube shuts divert aqueous from the anterior chamber to the subconjunctival space. Like a trabeculectomy, this creates a filtering bleb and the effectiveness and risks are similar to trabeculectomies. Three main types of tubes are available. They are the Ahmed, Molteno and Baerveldt (Figure 4). The Ahmed tube has a valve while the Baerveldt and Molteno are non-valved. Tubes will reduce the IOP between 30% to 50%.36
After a tube procedure, the patient should be followed at day one and then weekly for one month. The patient can then be followed every two weeks until the tube opens and the bleb fills. This typically occurs when the suture dissolves. One possible advantage of a Baerveldt or Molteno is that the bleb does not open right away. Thus, any inflammatory-related cytokines are usually greatly reduced by the time the bleb opens, thus reducing fibrosis and scarring. One disadvantage is that the IOP does not typically drop to its full potential until the bleb opens.
Postoperative complications include hyphema, hypotony, choroidal effusion, bullous keratopathy and double vision. Diplopia may occur because of extraocular muscle involvement of the tube. This often is temporary and can managed with prisms until resolution. Long-term tube complications include increased IOP due to fibrosis.37
If the bleb is excessively high, this might indicate scar tissue formation around the plate. If the bleb is low, that might indicate a blockage in the tube.
During the early postoperative period, a Molteno bleb will often be flat as a vicryl dissovable suture is used around the shaft of the tube. Once the suture dissolves, then aqueous flows into the bleb and fills it. The tube may be blocked by vitreous, fibrin, or iris tissue. Sometimes the blockage can be lasered open using a YAG or argon. If the tube is blocked by a blood clot, an injection of a tissue plasminogen activator may be helpful.
Optometrists have an essential role to play in managing today’s glaucoma patients. As the profession takes on a more prominent role in the comanagement space, we must make ourselves familiar with all of the options to our patients so we can be prepared to render the best postoperative care possible.
Dr. Cymbor is a partner at Nittany Eye Associates in State College, PA. He is also the co-director of the Glaucoma Institute of State College and a member of the Optometric Glaucoma Society.
1. Weinreb R, Aung T, Medeiros F. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901-11. 2. Vera V, Naqi A, Belovay G, et al. Dysphotopsia after temporal versus superior laser peripheral iridotomy: a prospective randomized paired eye trial. Am J Ophthalmol. 2014 May 1;157(5):929-35. 3. Srinivasan K, Zebardast N, Krishnamurthy P, et al. Comparison of new visual disturbances after superior versus nasal/temporal laser peripheral iridotomy: a prospective randomized trial. Ophthalmology. 2018;125(3):345-51. 4. Azuara-Blanco A, Burr J, Ramsay C, et al. EAGLE Study Group. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. The Lancet. 2016 Oct 1;388(10052):1389-97. 5. Weinand F, Althen F. Long-term clinical results of selective laser trabeculoplasty in the treatment of primary open angle glaucoma. Eur J Ophthalmol. 2006;16(1):100-4. 6. Skalicky S, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012 Jan;153(1):1-9. 7. Tsai J. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology. 2009;116(11):S30-6. 8. Realini T. Selective laser trabeculoplasty for the management of open-angle glaucoma in St. Lucia. JAMA Ophthalmology. 2013 Mar;131(3):321-7. 9. Katz L, Steinmann W, Kabir A, et al. Selective laser trabeculoplasty versus medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma. 2012 Sep;21(7):460-8. 10. Groth S. Steroids after laser trabeculoplasty (SALT) trial: Impact of short-term use of anti-inflammatory treatment on the efficacy of SLT. Presented at: The American Glaucoma Society annual meeting, Feb. 28 to March 4, 2018; New York. 11. Harasymowycz P, Papamatheakis D, Latina M, et al. Selective laser trabeculoplasty (SLT) complicated by intraocular pressure elevation in eyes with heavily pigmented trabecular meshworks. Am J Ophthalmol. 2005;139:1110-3. 12. Bettis D, Whitehead J, Farhi P, et al. Intraocular pressure spike and corneal decompensation following selective laser trabeculoplasty in patients with exfoliation glaucoma. J Glaucoma. 2016 Apr;25(4):e433-7. 13. Lai J, Chua J, Tham C, Lam D. Five-year follow up of selective laser trabeculoplasty in Chinese eyes. Clin Exp Ophthalmol. 2004 Aug; 32(4):368-72. 14. Rosenfeld E, Shemesh G, Kurtz S. The efficacy of selective laser trabeculoplasty versus argon laser trabeculoplasty in pseudophakic glaucoma patients. Clin Ophthalmol. 2012;6:1935-40. 15. Wong M, Lee J, Choy B, et al. Systematic review and meta-analysis on the efficacy of selective laser trabeculoplasty in open-angle glaucoma. Surv Ophthalmol. 2015 Jan-Feb;60(1):36-50. 16. Lavia C, Dallorto L, Maule M, et al. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: A systematic review and meta-analysis. PloS One. 2017 Aug 29;12(8). 17. Armaly M. Effect of corticosteroids on intraocular pressure and fluid dynamics. II. The effect of dexamethasone in the glaucomatous eye. Arch Ophthalmol. 1963 Oct;70:492-9. 18. Coban-Karatas M, Sizmaz S, Altan-Yaycioglu R, et al. Risk factors for intraocular pressure rise following phacoemulsification. Indian J Ophthalmol. 2013 Mar;61(3):115. 19. Radcliffe N, Kahook M. Endocyclophotocoagulation. Essectials Glaucoma Surg. 2012;2012:211-5. 20. Roberts S, Mulvahill M, SooHo J, et al. Efficacy of combined cataract extraction and endoscopic cyclophotocoagulation for the reduction of intraocular pressure and medication burden. Inter J Ophthalmol. 2016;9(5):693-8. 21. Berke S, Sturm R, Caronia R, et al. Phacoemulsification combined with endoscopic cyclophotocoagulation (ECP) in the management of cataract and medically controlled glaucoma: a large, long term study. Presented at the AGS 16th Annual Meeting, 2006 Mar 4. 22. Malvankar-Mehta M, Iordanous Y, Chen Y, et al. iStent with phacoemulsification versus phacoemulsification alone for patients with glaucoma and cataract: A meta-analysis. PLoS One. 2015;10(7):e0131770. 23. Chang D, Donnenfeld E, Katz L, et al. Efficacy of two trabecular micro-bypass stents combined with topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 3-year follow-up. Clinical ophthalmology (Auckland, NZ). 2017;11:523. 24. Vlasov A, Kim WI. The efficacy of two trabecular bypass stents compared to one in the management of open-angle glaucoma. Military medicine. 2017 Mar 1;182(suppl 1):222-5. 25. Craven E, Katz L, Wells J, Giamporcaro J. iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339-45. 26. Dorairaj S, Kahook M, Williamson B, et al. A multicenter retrospective comparison of goniotomy versus trabecular bypass device implantation in glaucoma patients undergoing cataract extraction. Clinical Ophthalmology (Auckland, NZ). 2018;12:791. 27. Jordan J, Wecker T, van Oterendorp C, et al. Trabectome surgery for primary and secondary open angle glaucomas. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2013 Dec 1;251(12):2753-60. 28. Mosaed S. The first decade of global trabectome outcomes. Clin Surg Ophthalmol. 2014;32:1. 29. Vold S, Ahmed II, Craven E, et al. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016 Oct 1;123(10):2103-12. 30. Pillunat L, Erb C, Jünemann A, Kimmich F. Micro-invasive glaucoma surgery (MIGS): A review of surgical procedures using stents. Clin Ophthalmolo. 2017 Aug;11:1583-1600. 31. Pérez-Torregrosa V, Olate-Pérez Á, Cerdà-Ibáñez M, et al. Combined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisions. Archivos de la Sociedad Española de Oftalmología (English Edition). 2016 Sep;91(9):415-21. 32. Sheybani A, Lenzhofer M, Hohensinn M, et al. Phacoemulsification combined with a new ab interno gel implant to treat open-angle glaucoma: a pilot study. J Cataract Refract Surg. 2015;41:1905-9. 33. Zhou M, Wang W, Huang W, Zhang X. Trabeculectomy with versus without releasable sutures for glaucoma: a meta-analysis of randomized controlled trials. BMC ophthalmology. 2014 Dec;14(1):41. 34. O’brart D, Shiew M, Edmunds B. A randomised, prospective study comparing trabeculectomy with viscocanalostomy with adjunctive antimetabolite usage for the management of open angle glaucoma uncontrolled by medical therapy. Br J Ophthalmol. 2004 Aug;88(8):1012-7. 35. Jampel H, Musch D, Gillespie B, et al. Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS). Am J Ophthalmol. 2005 Jul 1;140(1):16-22. 36. Riva I, Roberti G, Katsanos A, et al. A review of the Ahmed Glaucoma Valve Implant and comparison with other surgical operations. Advances in Therapy. 2017 Apr;34(4):834-47. 37. Syed H, Law S, Nam S, et al. Baerveldt-350 implant versus Ahmed valve for refractory glaucoma: a case-controlled comparison. J Glaucoma. 2004 Feb;13(1):38-45. |

