 |
Q: List the blood product options for dry eye patients. What are their advantages and disadvantages? What contraindications and risks do they carry?
“Dry eye disease is an increasingly common ophthalmic condition that negatively impacts the quality of life of millions worldwide,” says Andrew Fischer, OD, of Professional Eyecare Associates in Indiana. As our understanding of this disease continues to expand, so does the availability of our treatment options, he adds.
Blood-based dry eye treatments have increased in popularity and aided many dry eye patients for whom “traditional” treatment methods may have been unable to provide adequate relief. Blood-based products can be drawn from the patient themselves (autologous) or from a donor (allogeneic) and can be prepared in various treatment forms, including serum eye drops and platelet-derived eye drops. Blood-based drops are indicated for moderate to severe dry eye, persistent epithelial defects, graft-vs.-host disease, recurrent corneal erosions, neurotrophic keratitis and limbal stem cell deficiency, to name a few.
In short, blood-derived dry eye treatments help accelerate corneal epithelial cell healing by increasing the growth factor available to the ocular surface. While there is an abundance of clinical research supporting the use of blood-based eye drops for dry eye, this treatment is not FDA-approved; therefore, there is not a universally accepted standardized protocol preparation.
 |
|
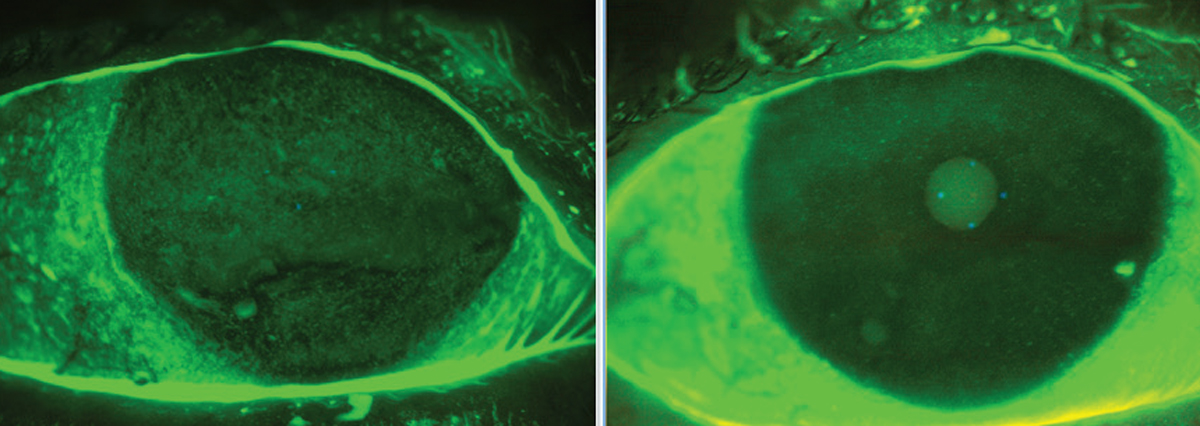
This dry eye patient experienced significant relief after approximately 12 weeks of blood-based treatment. Click image to enlarge. |
Serum Drops
This is perhaps the most common blood-based product used in dry eye therapy, says Dr. Fischer. To prepare serum eye drops, a sample of blood is obtained, allowed to clot at room temperature and centrifuged to separate the blood cell components from the serum. The serum is then collected and diluted with a balanced salt solution to prespecified concentrations, most commonly 20% and 50% serum by volume, but higher concentrations can be prepared.
These drops have potential limitations, as blood serum can contain pro-inflammatory cytokines, especially in patients with autoimmune diseases such as Sjögren’s syndrome or graft-vs.-host disease. For these patients, autologous serum may be counterproductive and lead to further degradation of the ocular surface.1
Platelet-derived Drops
Options in this category include platelet-rich plasma (PRP), platelet-rich growth factor (PRGF) and platelet lysate (PL). Unlike serum eye drops, these preparations contain blood platelets, which inherently have high levels of growth hormones, promoting wound healing and corneal epithelial regeneration.2
To produce platelet-based drops, an anticoagulant is added to a blood sample, which is then centrifuged and separated into platelet-poor plasma, PRP and red and white blood cells. The PRP is extracted and transferred into dropper bottles for use. Unlike serum eye drops, PRP drops aren’t diluted. PRGF can be produced from PRP by adding calcium chloride, which significantly increases growth factor concentration. PL is derived from PRP, diluted to 30% by volume and frozen then thawed to increase growth factor concentration due to platelet thermolysis.
Takeaways
Contraindications to autologous blood products are largely relative, as the product is ultimately derived from the patient’s own body; however, blood infections, anemia and clotting disorders can be considered contraindications. In these cases, allogenic samples are an option.
Blood-derived dry eye treatments are especially useful in patients who are sensitive to preservatives or request a more holistic approach to dry eye treatment. While the TFOS DEWS II considers blood-based therapy a third-line treatment, it may be worth taking into consideration earlier in the process, especially in patients who could benefit from the robust healing properties of natural growth factors
Dr. Shovlin, a senior optometrist at Northeastern Eye Institute in Scranton, PA, is a fellow and past president of the American Academy of Optometry and a clinical editor of Review of Optometry and Review of Cornea & Contact Lenses. He consults for Kala, Aerie, AbbVie, Novartis, Hubble and Bausch + Lomb and is on the medical advisory panel for Lentechs.1. Ma IH, Chen LW, Tu WH, et al. Serum components and clinical efficacies of autologous serum eye drops in dry eye patients with active and inactive Sjogren syndrome. Taiwan J Ophthalmol. 2017;7(4):213-20.
2. Alio JL, Rodriguez AE, Ferreira-Oliveira R, et al. Treatment of dry eye disease with autologous platelet-rich plasma: a prospective, interventional, non-randomized study. Ophthalmol Ther. 2017;6(2):285-93.

