 |
While many patients with glaucoma experience ocular surface disease (OSD) issues, adding another bottle to a glaucoma patient already on chronic medications could result in a loss of compliance and the potential for intraocular pressure rise. However, not treating the OSD can also lead to patients discontinuing their glaucoma medications secondary to pain and discomfort. How do we solve this dilemma?
Implications of Topical Glaucoma Agents on the Ocular Surface
A key clinical consideration for topical antiglaucoma medications is their potential to cause or exacerbate OSD. Though they are a first-line treatment in glaucoma therapy, prostaglandin analog have been associated with a high rate of meibomian gland dysfunction.1 It is critical that eyecare practitioners (ECPs) understand the chronic effects of these drugs for patients, and consequently, for patient adherence.
An additional contributor to OSD in patients with glaucoma-induced dry eye is chronic exposure to formulations containing preservatives such as benzalkonium chloride (BAK). Nonspecific to any class of antiglaucoma agents, BAK is an inflammatory element that can damage the ocular surface epithelium, disrupt the tear film and even lead to punctate keratitis of the cornea.2 Some preservative-free glaucoma medications are an option, although payor coverage is variable. These include Iyuzeh (latanoprost 0.005%, Thea Pharma), Zioptan (tafluprost 0.0015%) and Cosopt PF (dorzolamide/timolol ophthalmic solution 2%/0.5%). Another option, such as selective laser trabeculoplasty, may not address ocular surface symptoms but can help reduce drop burden.3 MIGS—if cataract surgery is needed—or injecting bimatoprost SR (Durysta, Abbvie) are other options; however, some patients may not be candidates or not have access.
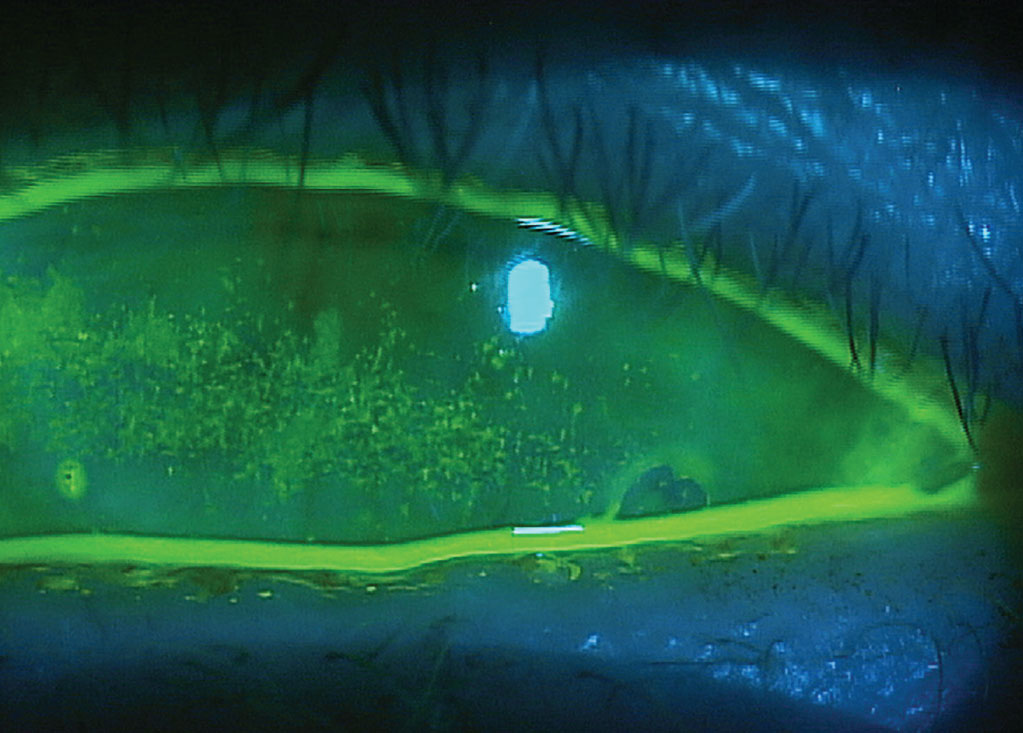 |
|
Glaucoma-induced dry eye in a patient using multiple BAK-preserved therapies. Click image to enlarge. |
Owing to their side effect profile, topical antiglaucoma agents often present a challenge to medication adherence. Patients may be noncompliant or even self-discontinue their chronic glaucoma medications due to discomfort, irritation and/or blurred vision.4 Beyond reinforcing patient education, ECPs should understand that management of dry eye disease (DED) in patients with glaucoma require a different set of treatment considerations.
Shift in Clinical Approach
The neurotrophic effects from chronic use of proinflammatory drops and preservatives can be a differentiating factor in how patients present with glaucoma-induced dry eye. Whereas DED symptoms commonly include dryness and irritation in the general population, patients with chronic glaucoma typically report blurred vision, irritation and painful discomfort, specifically when instilling antiglaucoma drops. Another set of unique differences in this group is that patients often have pre-existing conjunctival hyperemia from glaucoma drops and/or report photophobia.
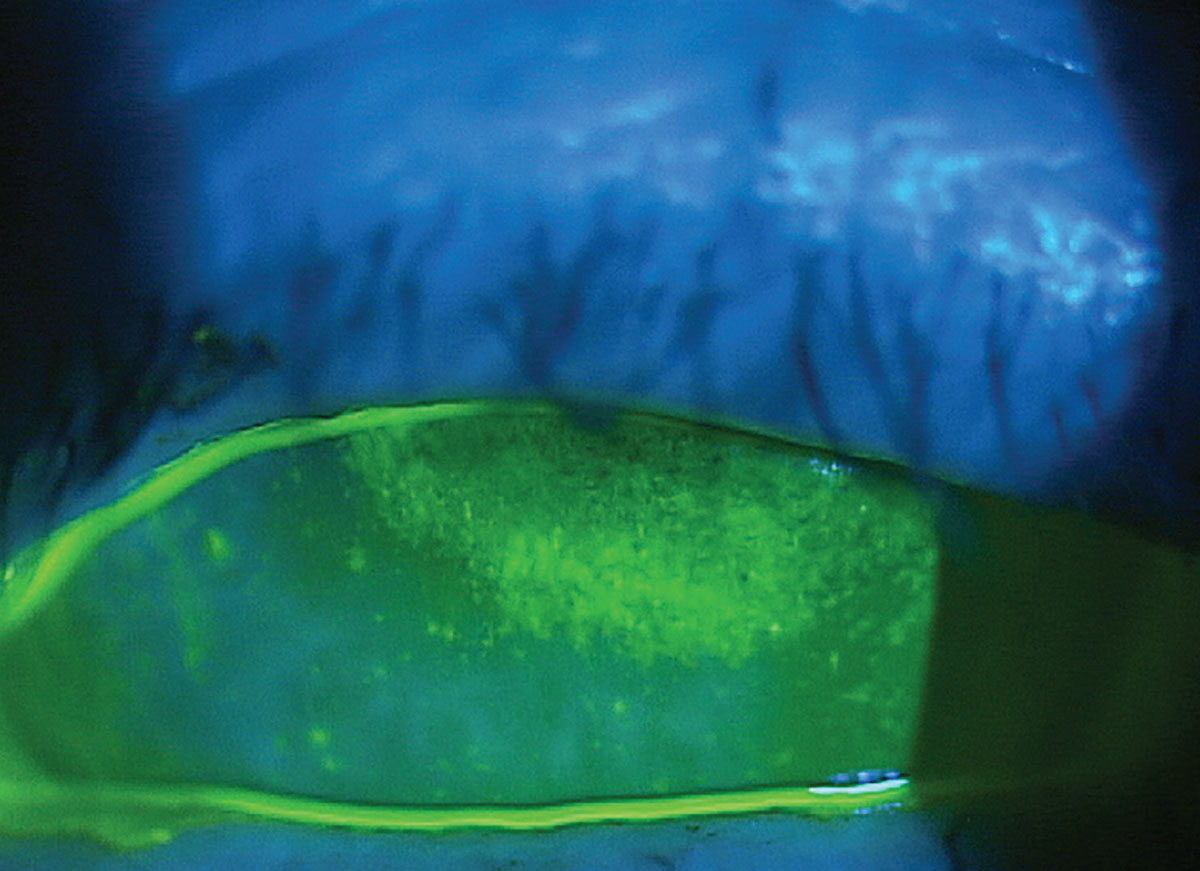 |
|
Neurotrophic keratitis in a patient with longstanding use of topical glaucoma agents. Click image to enlarge. |
While steroid drops are often used in dry eye patients with superficial punctate keratitis, long-term use of steroid drops would be a contraindication in patients with glaucoma. Yet ECPs should also think broadly about reducing the eye drop burden, or at the very least, the implications of an additional eye drop in a glaucoma regimen that already incorporates multiple daily administrations. In-office procedures can be an effective way to manage patients with glaucoma-induced OSD.
If targeting the meibomian glands, thermal expression procedures such as intense pulse light therapy and low-level laser therapy or thermal expression procedures (TearClear, iLux or LipiFlow) help avoid noncompliance. Alternatively, punctal plugs can be an excellent treatment for patients with DED to help with dry eye and have also been shown to increase the effectiveness of glaucoma medications.5,6 To avoid irritation on the ocular surface, consider punctal plugs that reside in the vertical canal only or fill the entire lacrimal system. Such examples are the 180 dissolving punctal plugs with tapered ends (Oasis Medical), form-fitting punctal plugs (Oasis Medical) or lacrimal fillers (Lacrifill, Nordic Pharmaceuticals).
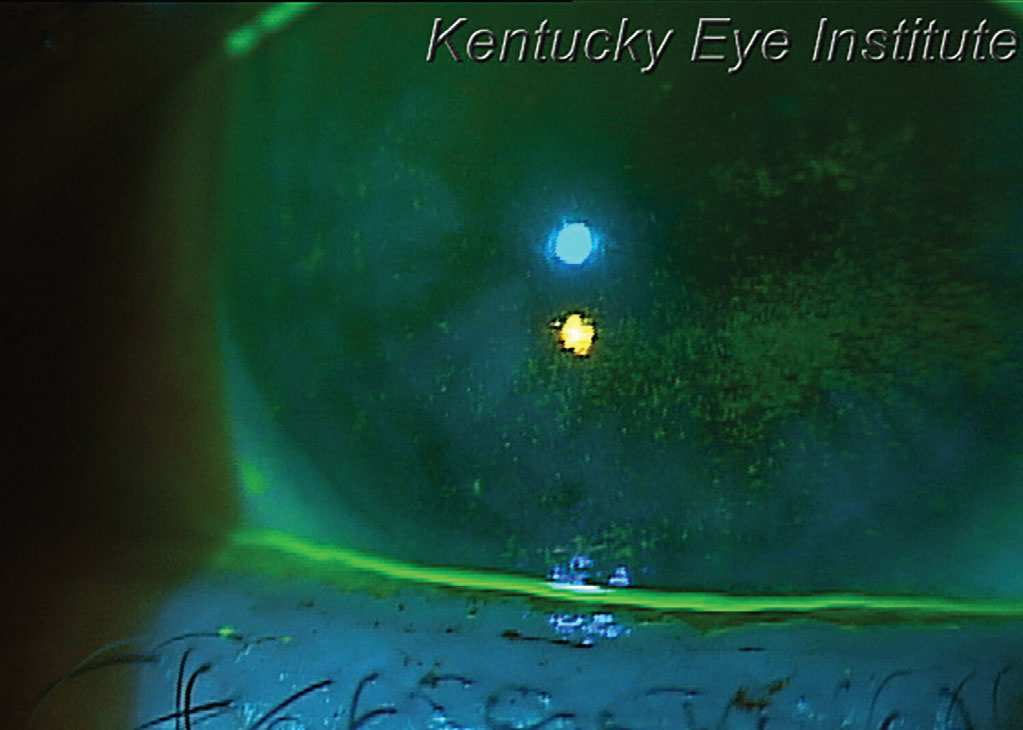 |
|
This patient with glaucoma-induced dry eye and a thin tear meniscus could benefit from punctual occlusion. Click image to enlarge. |
Avoiding the Ocular Surface
Prescription therapies such as varenicline (Tyrvaya, Viatris Pharmaceuticals) are neurostimulators that use the nasal route to promote a basal tear reflex, replenishing all three layers of the team film. External stimulators iTear 100 (Olympic Ophthalmics) can also be ordered that also have good success in stimulating basal tear production when needed.
Amniotic Membrane
In light of some of the current limitations of inflammation treatment, amniotic membrane can be an excellent in-office option.7 For example, cryopreserved material (Prokera, BioTissue) maintains key anti-inflammatory components that form the heavy-chain, high molecular weight hyaluronic acid/pentraxin 3 (HC-HA/PTX 3) matrix for corneal wound healing.8 Patients with a trabeculectomy bleb may not be suitable for treatment with CAM in part due to irritation concerns. In this case, there are dehydrated formulations that maintain many anti-inflammatory components and growth factors (e.g., Apollo, Atlas Medical). The rapid effects (typically inserted for three to four days in the case of cryopreserved amnion) make it a favorable option in glaucoma-induced dry eye management.
More commonly, patients with glaucoma-induced dry eye who are affected through the meibomian glands stand to benefit from a targeted approach.
Implementing Best Treatment Practices
Like any chronic disease, OSD is more effectively managed with early treatment. In cases of glaucoma-induced OSD, the cause or stimuli of dry eye symptoms will typically remain in place for the rest of many patients’ lives with the use of IOP-lowering drops. Early disease identification combined with aggressive treatment that minimizes the use of drops and preservatives is an ideal way of managing this large subset of patients.
Dr. Karpecki is the director of Cornea and External Disease for Kentucky Eye Institute, associate professor at KYCO and medical director for Dry Eye Institutes of Kentucky and Indiana. He is the Chief Clinical Editor for Review of Optometry and chairman of the affiliated New Technologies & Treatments conferences. A fixture in optometric clinical education, he provides consulting services to a wide array of ophthalmic clients. Dr. Karpecki’s full disclosure list can be found here.
1. Mocan MC, Uzunosmanoglu E, Kocabeyoglu S, et al. The association of chronic topical prostaglandin analog use with meibomian gland dysfunction. J Glaucoma. 2016;25(9):770-4. 2. Boso ALM, Gasperi E, Fernandes L, et al. Impact of ocular surface disease treatment in patients with glaucoma. Clin Ophthalmol. 202014:103-11. 3. Ma AK, Lee JH, Warren JL, Teng CC. GlaucoMap - distribution of glaucoma surgical procedures in the United States. Clin Ophthalmol. 2020;14:2551-60. 4. Potop V. Effects of preservatives in anti-glaucoma agents on the ocular surface. Oftalmologia. 2009;53(2):92-6. 5. Huang TC, Lee DA. Punctal occlusion and topical medications for glaucoma. Am J Ophthalmol. 1989;107(2):151-5. 6. Opitz DL, Tung S, Unsun S, et al. Silicone punctal plugs as an adjunctive therapy for open-angle glaucoma and ocular hypertension. Clin Exp Optom. 2011;94(5):438-42. 7. Morkin M, Hamrah, P. Efficacy of self-retaining cryopreserved amniotic membrane for treatment of neuropathic corneal pain. Ocul Surf. 2018;16(1):132-8. 8. Tan EK, Cooke M, Mandrycky C, et al. Structural and biological comparison of cryopreserved and fresh amniotic membrane tissues. J Biomaterial Tissue Engineering. 2014,4(5):379-8. |

