Glaucoma Pearls & PitfallsIn the July 2024 issue of Review of Optometry, our 30th annual Glaucoma Report, seasoned ODs share tips on using modern tools, technology and knowledge to provide top-notch care to your patients with this chronic condition. Check out the other articles featured in this issue:
|
As you approach the door to greet your next patient, your technician briefs you on the situation: a newcomer seeking reassurance about their glaucoma. But there’s a twist—a daunting 23-page handwritten record awaits your scrutiny. Steadying your breath, you swing open the door, ready to tackle the challenge head-on.
Inheriting a patient with a pre-existing glaucoma diagnosis can feel like stepping into the middle of a complex story. While their medical history provides valuable context, their current experience with the disease, anxieties and treatment adherence may take several visits to uncover. This initial encounter presents a unique challenge for clinicians and requires a nuanced approach to navigate their current needs while respecting existing management plans.
Records Review
When assuming the care of new patients, whether they’re suspected of having glaucoma or are already undergoing treatment, it’s crucial to thoroughly review all available past information.
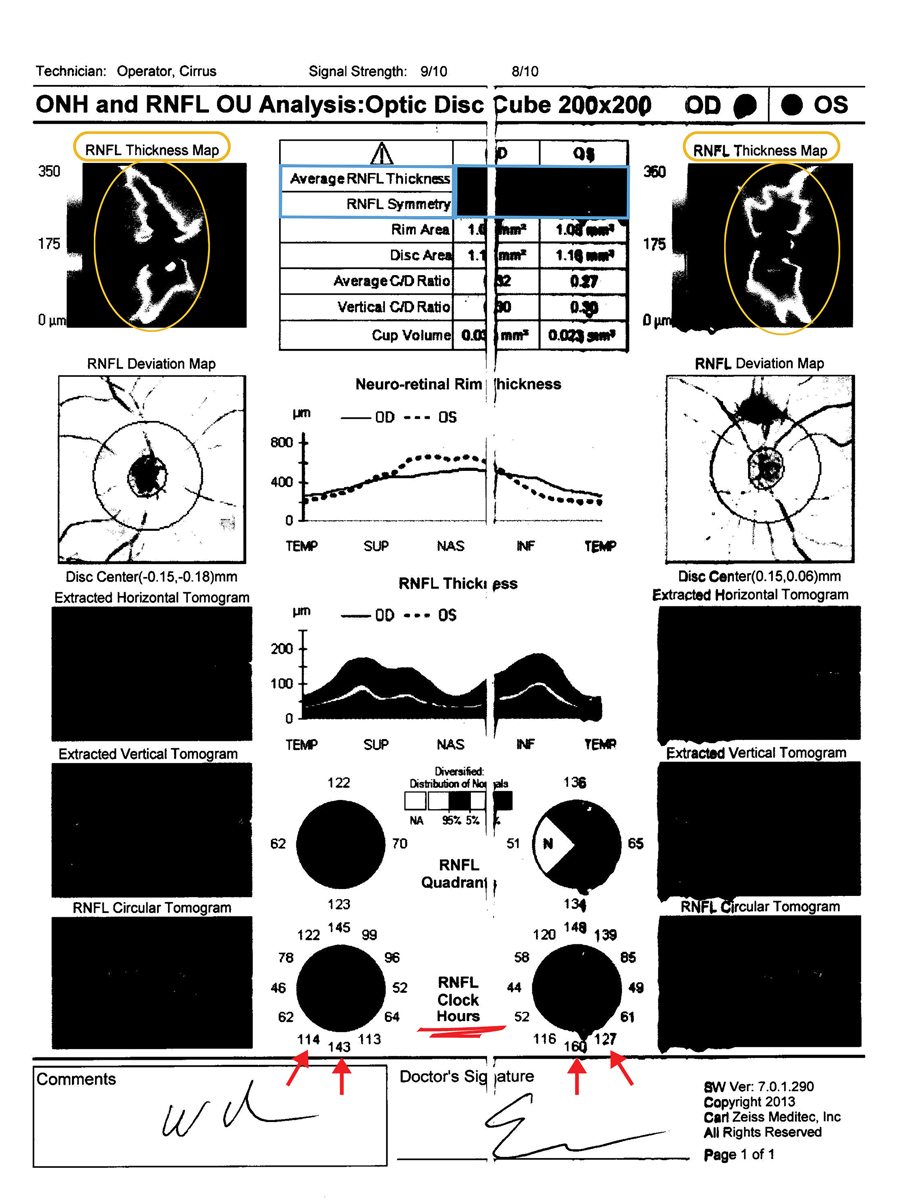
Encouraging patients to sign medical records release forms can help in obtaining their previous records and test results, yet there are still some limitations. One challenge is the lack of consistency among practitioners in how they interpret test results and document their findings, which can complicate the continuity of care. In optometry and ophthalmology, there’s a broad range of electronic health records systems, and some practitioners still rely on paper charts, necessitating ongoing maintenance of physical copies over the years. Relying on paper charts can lead to issues such as legibility challenges and printing errors, which makes it harder to interpret the information. Additionally, when practitioners receive information via fax, the quality of black and white images may restrict the optical coherence tomography (OCT) details available, including average retinal nerve fiber layer (RNFL) thickness, symmetry and thickness and deviation maps (Figure 1). Speaking with the patient’s pharmacists may give key insight to compliance, information about their past providers and expected treatment regimen.
 |
|
Fig. 1. The limitations of black and white printed records of RNFL thickness and deviation maps as well as average RNFL thickness and symmetry make this faxed copy of little use. RNFL clock hours can still be interpreted and compared to future testing. Click image to enlarge. |
Glaucoma is a multifactorial disease in which demographics such as age, race, sex, family history, geographic location, ocular perfusion pressure and systemic disease act as risk factors for development.1 Other important factors include a careful angle assessment and whether there are characteristics that might indicate aggressive glaucomas like pseudoexfoliation or pigmentary. Additionally, understanding how to use objective elements including intraocular pressure (IOP), pachymetry and corneal biomechanics, OCT and visual fields is vital for a confident diagnosis and management.
IOP
In evaluating glaucoma patients for the first time, understanding this measurement is critical, as it’s the primary modifiable risk factor in the progression of glaucomatous optic neuropathy, with all treatments aiming to lower it. IOP fluctuates throughout the day due to circadian rhythms.2
Despite patients typically being seen two to six times a year, this provides only a limited number of data points. For instance, even if a patient is seen four times a year, we’re only measuring IOP for a mere four seconds out of over 31 million seconds in a year. This scarcity of data can pose challenges when initiating or adjusting therapies, particularly since the method we use to measure IOP, such as the Goldman applanation tonometer, introduced in the 1950s, has known inaccuracies.3 Factors such as corneal thickness, previous surgeries, edema or astigmatism can affect readings.4 Inconsistent readings can also arise between doctors and technicians.
Identifying the maximum IOP (Tmax) remains a crucial step for assessing open-angle glaucoma (OAG) risk due to its ability to indicate large diurnal fluctuations.5 When searching old records, Tmax should be a priority. Other factors include which instrument was used to measure IOP and the time of day tested. Additionally, regulating the autonomic nervous system is vital for accurate IOP measurements. During the Valsalva maneuver, there’s heightened autonomic nervous system activity, affecting heart rate variability and possibly blood flow, which can raise IOP.6 Recent findings suggest that incorporating “365 breathing” into glaucoma treatment, along with standard therapies, leads to significantly lower IOP and cortisol levels and improved autonomic regulation.7
To enhance the reliability of IOP measurements, it’s advisable to take multiple readings and calculate an average, assess the tear film height (0.2mm to 0.5mm average) to ensure there is neither an excess or insufficient fluorescein before measurement, ensure patients don’t hold their breath and be cautious of any orbital pressure when holding eyelids during measurements.
 |
|
Fig. 2. Robust RNFL OD and OS with no progression. Click image to enlarge. |
Pachymetry and Corneal Biomechanics
Average central corneal thickness (CCT) as measured by pachymetry is estimated to be between 540µm to 550µm.8 Thicker corneas tend to overestimate measured IOP, while thinner corneas tend to underestimate IOP. Pachymetry has also been shown as an independent risk factor for the development of glaucoma, with lower CCT being a larger risk factor for glaucomatous progression.9
Other corneal biomechanics are important to consider, including the influence of corneal hysteresis (CH), which determines the cornea’s ability to dissipate energy. Eyes with lower CH have a higher likelihood of developing glaucoma and a faster rate of progression by visual field.10,11
OCT
This technology has become a main tool in monitoring glaucomatous progression and enjoys widespread acceptance.12 While OCTs from prior providers may give additional information, new scans will generally need to be performed because of both manufacturer nuance and baselining for future progression analysis.
Using OCT as a differentiator of non-glaucomatous vs. glaucomatous optic neuropathy (NGON vs. GON) requires attention to several details. While assessing the optic nerve head (ONH), cup-to-disc asymmetry <0.2 in the absence of disc asymmetry remains a hallmark of glaucomatous change.13 The cup-to-disc ratio relative to optic nerve size is also of significance with superficial optic disc areas averaging from 2.1mm2 to 2.35mm2 on OCT in a normal Caucasian population.14 Large discs will physiologically correspond with larger cups, and smaller discs are more likely to have smaller cups. RNFL thickness and deviation maps are especially useful in diagnosing early glaucomatous slit defects.
While OCT is commonly used, there are weaknesses to this technology such as having no consensus on what constitutes progression.15 There is also no general agreement on the number, frequency or spacing of scans to detect progression. As a result, over- or underidentifying change can have significant unintended consequences on patient outcomes.16
It is important to acknowledge that clinical information obtained from OCT is relative to the reference database, which may or may not align with a given patient’s specific demographics (e.g., age, race, disc area, axial length).17 The “ISNT rule” characteristically describes normal optic nerve disc rim thickness in which divided sectors expected thicknesses are as follows: inferior (I) is greater or equal to superior (S) greater or equal to nasal (N) greater or equal to temporal (T).18 Beneficially, it is independent of race and is useful for differentiating glaucomatous optic nerves. When using the ISNT rule as it applies to OCT, one may need to consider nerves with oblique insertion, segmental disc hypoplasia and high myopia or extensive peripapillary atrophy.19
Macular changes can be especially useful to assist in correlating RNFL defects. Ganglion cell analysis (GCA) provides the ability to divide the central macula into critical zones. The presence of a temporal raphe sign is highly indicative of GON.20 It has been suggested that macular thickness varies with respect to various glaucomatous risk factors. Among patients with pseudoexfoliation syndrome (PXS) and ocular hypertension (OHT), the inner retinal layers appear most thin in the patients with ocular hypertensive PXS, with normotensive PXS and OHT groups following in a respective order.21
However, not all arcuate-shaped RNFL loss indicates glaucomatous progression. Multiple macular RNFL defects in the absence of ONH cupping may be more suggestive of hypertensive or diabetic changes in non-glaucomatous eyes.22 Confounders may exist in this region including areas where prior vitreomacular traction then released as well as NGON (uveitis, ischemic optic neuropathy, etc.) induced RNFL edema with subsequent resolution.23
 |
|
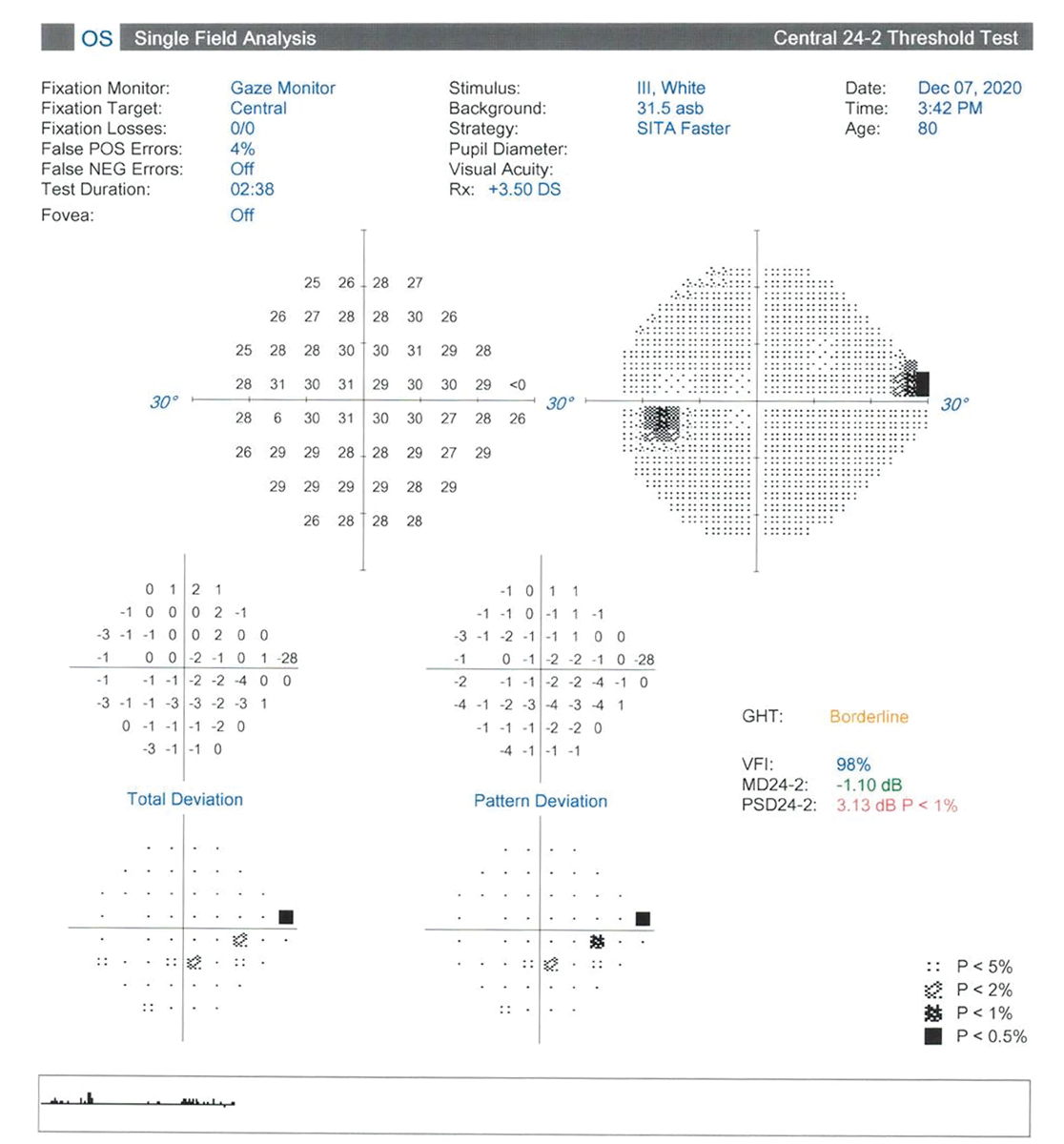
Fig. 3. Superior nasal defect that falls short of cluster criteria. Click image to enlarge. |
Visual Fields
Functional testing by visual fields is subjective, time-consuming and too often unreliable. Despite those limitations, they often help determine whether the patient has glaucoma or comorbidity. Scotomas (in arcuate or isolated fashion), nasal steps and generalized depressions are most likely seen in glaucoma.24 Studies suggest that performing multiple visual fields on the same day (frontloading) may enhance reliability in testing and yield more confident diagnosis.25 Past vasculopathies can confound glaucoma diagnosis. While fields from prior providers may add additional insight, like with OCTs, new fields should be taken to help with future progression analysis.
While the World Glaucoma Association recommends at least four visual fields in the first two years and possibly six if the patient is at risk for rapid progression, greater than 75% of glaucoma patients receive less that one field per year.26,27
Making Decisions
In many scenarios, the optometrist must decide: should treatment be continued, changed or discontinued altogether? Several factors may arise to lead practitioners to one decision or another.
Case 1: Continue Treatment. An 83-year-old woman with Alzheimer’s reported to the clinic accompanied by her husband. She was previously diagnosed with primary open-angle glaucoma (POAG) by a prior optometrist who had since retired. She was being treated with Xalatan (latanoprost, Pfizer) qhs OU. Her husband (who was also diagnosed with glaucoma) had expressed concerns regarding compliance due to her resistance to his administration of drop therapy. He also expressed the patient could not sustain long office visits without agitation.
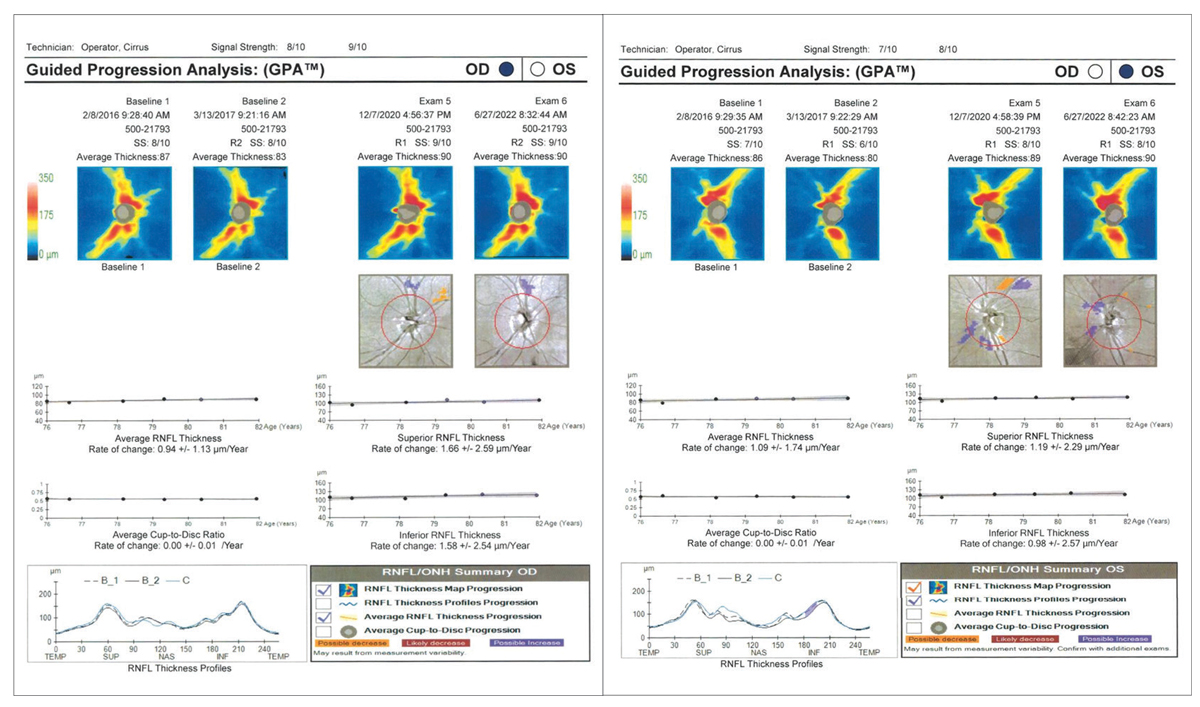
The patient’ s last Humphrey visual field (HVF) was performed in 2020 without glaucomatous defects. Their last OCT was performed in 2022 and demonstrated excellent reliability with robust RNFL on all clock hours and an average RNFL thickness of 90um and average cup-to-disc ratio of 0.57 for both OD and OS. Her IOP was measured at 12.0mm Hg OD and 14.0mm Hg OS with Ocular Response Analyzer at 9:46am. Her RNFL appeared robust for age-expected norms without suggestion of any slit or wedge defects (Figure 2). On RNFL GPA, the HVF was clear without glaucomatous defects in the right eye, while the left eye suggested a superonasal defect mimicking a nasal step on the gray scale but falling short of cluster criteria on the pattern deviation plot (Figure 3).
Modification or discontinuation of therapy could have been a possibility, but given the husband’s personal experience with glaucoma and the longevity of care with another provider, a mutual decision was made to continue with treatment to the best of their ability and monitor diurnal IOP closely. Had the patient’s glaucoma state been more severe or risked fixation, changing treatment options may have been considered. In this case, the patient and her husband were assured that quality of vision had a high chance of preservation given the patient’s structural stability. Keeping the treatment the same allows providers the opportunity to focus on building the patient’s trust and respect up to the same level they once had for their past providers.
 |
|
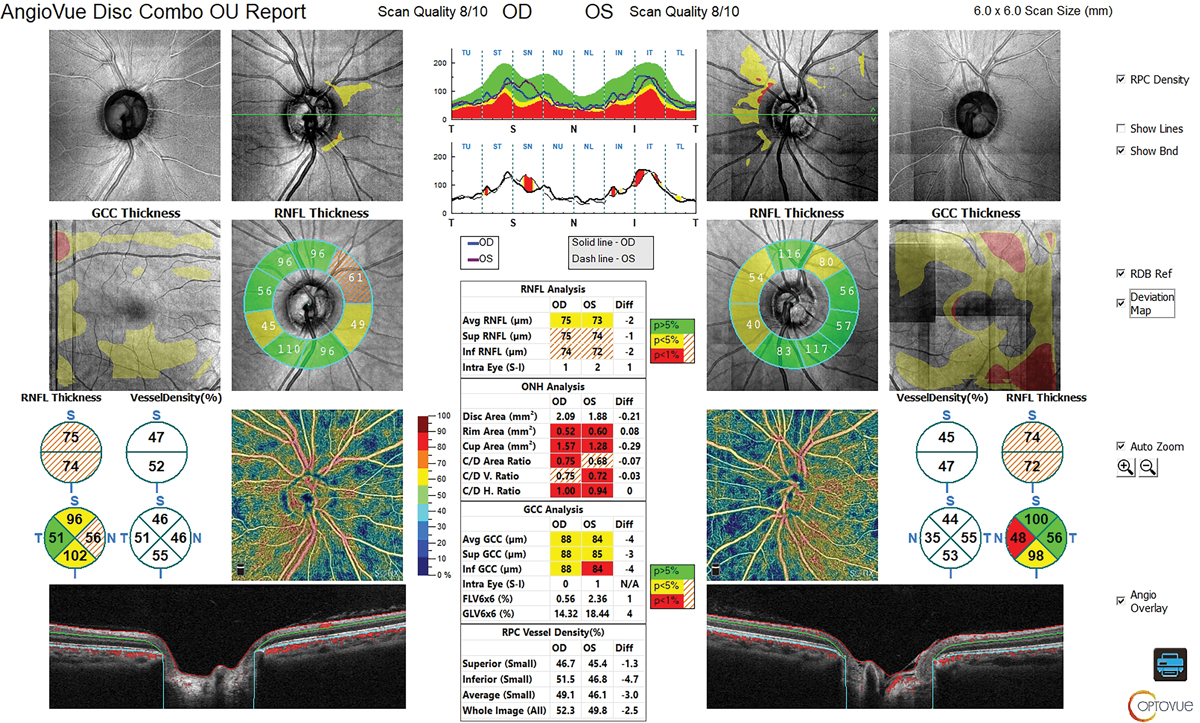
Fig. 4. RNFL and GCC of OCT show reasonable thickness without a glaucomatous pattern. Click image to enlarge. |
Future considerations could include therapeutic alternatives such as selective laser trabeculoplasty, Durysta (intracameral bimatoprost, AbbVie) and/or MIGS procedure(s). These all reduce the burden of compliance.
In general, removing the burden of medication compliance also reduces risk for falls and motor vehicle accidents. Fall risk in the elderly population has been positively correlated with visual field loss secondary to glaucoma.20 Elderly with glaucoma were 1.65-times more likely to be involved in a motor vehicle collision than elderly without glaucoma when visual acuity and contrast were corrected for.21
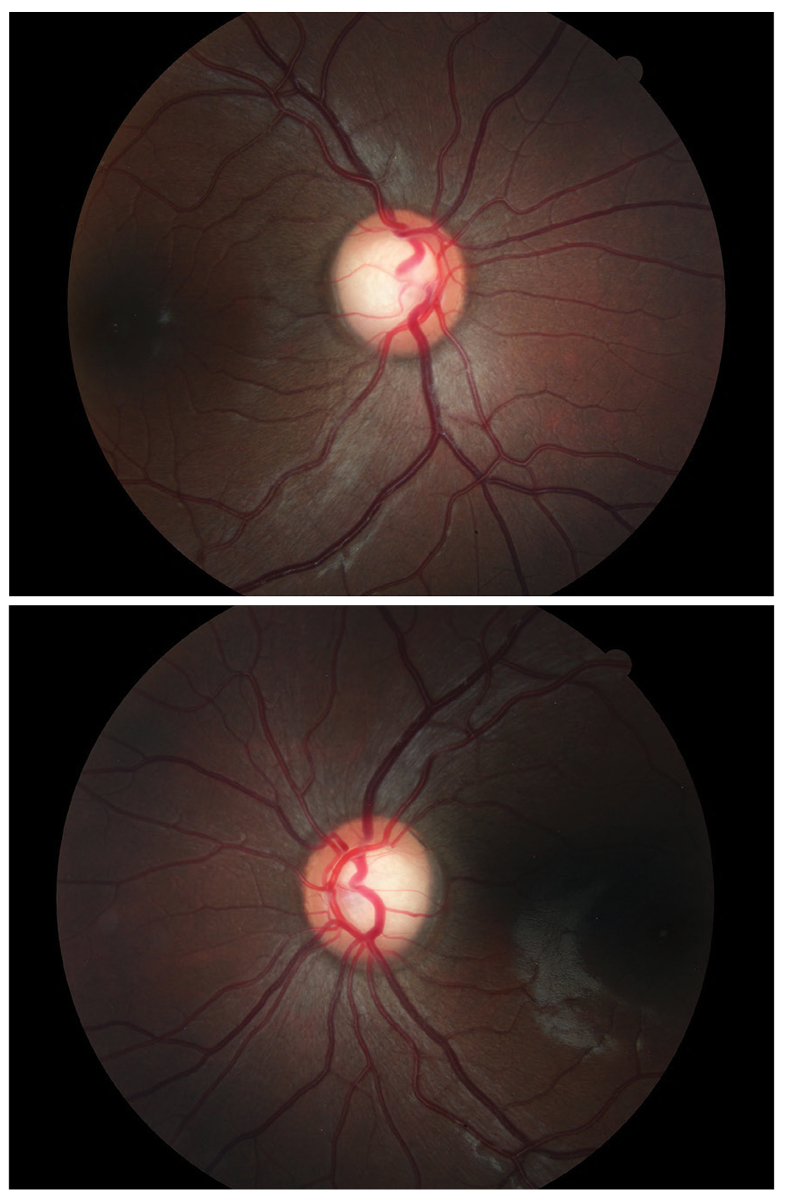
Case 2: Adjust Treatment. A 40-year-old Asian woman recently moved to the area and reported a previous diagnosis of glaucoma made by her general ophthalmologist. She reported excessive fatigue over the last several months. Her records indicate that she was diagnosed with POAG in 2018 and treated with 0.5% timolol bid OU. Previous records reported “large cupping with mild OCT dropout OU” with a Tmax of 19mm Hg OU and pachs of 577 and 605. Visual field report stated, “Central 30-2 Sita-fast presents as normal.” We were not able to get the actual structural or functional test results. While it is impossible to know by chart review exactly what led a previous provider to make the diagnosis, it appears that large cupping and possible OCT thinning were the key factors, despite the relatively young age, moderate to thick pachs and low Tmax.
 |
|
Fig. 5. Large cup with large disc. Click image to enlarge. |
At our exam, her corneal-corrected IOP was 16.0mm Hg OD and 17.1mm Hg OS. Her refractive error was -1.00D OD and OS correctable to 20/20. OCT revealed large cups and large discs. Her RNFL and ganglion cell complex (GCC) showed reasonable thickness with no evidence of glaucomatous structural loss (Figure 4). The visual field was unremarkable, with no evidence of glaucomatous functional loss. Gonioscopy revealed angles open to ciliary body with grade 1 pigmentation of trabecular meshwork and no synechiae.
We explained that either her glaucoma is extremely well controlled or that it is possible that she may not need treatment. We also explained that her timolol treatment may be contributing to her fatigue. We discussed using a different drop vs. a complete discontinuation of treatment. She felt most comfortable switching to a different drop, and we started her on tafluprost. One month later her corneal-corrected IOP decreased to 13.2mm Hg OD and 14.1mm Hg OS, and she reported a significant reduction in fatigue. We will continue to monitor every six months.
• Case 3: Discontinue Treatment. A 49-year-old African American woman who recently moved to the area reported a previous diagnosis of POAG with a family history of glaucoma (sister, mother and paternal grandmother). She brought in paper records from her previous general ophthalmologist. The records, while barely legible, indicate that she was diagnosed as a POAG suspect based on large cupping (cup-to-disc ratio listed as 0.7 OD and OS), an asymmetric IOP of 15mm Hg OD and 22mm Hg OS by Goldmann, family history and “possible progression by Heidelberg Retina Tomograph.” There was a notation of “gonio—open to CB.”
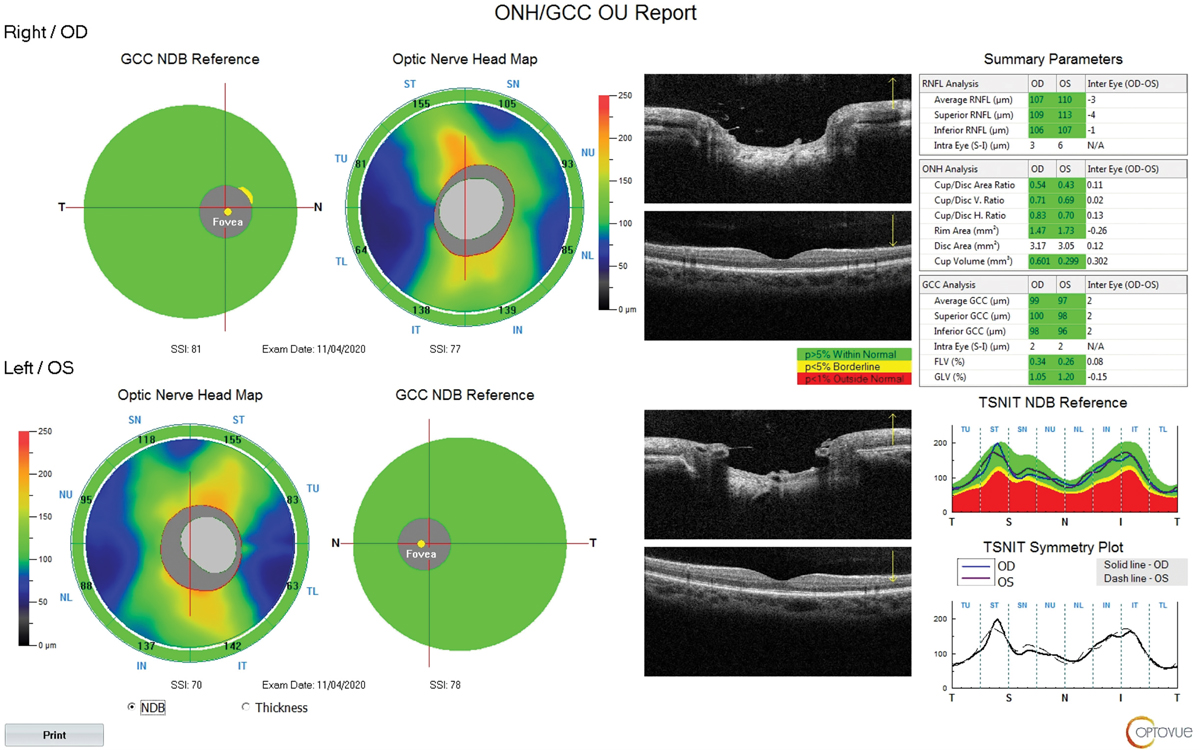
We did not receive any of the actual test results, and there were no test reports in the records we received. The patient was then started on bimatoprost qhs OU, which lowered the pressure to the low to mid-teens. The patient was followed for several years, with no further notes indicating progression.
At our exam, best-corrected visual acuity was 20/20 OD and OS. Her refractive error was -2.00 OD and -1.75 OS. Corneal compensated IOP by Ocular Response Analyzer was 11.8mm Hg OD and 13.7mm Hg OS on treatment. Corneal pachymetry was 489µm OD and 488µm OS. Corneal hysteresis was 10.8 and 10.7. Cup-to-disc ratio was estimated at 0.7 OD and OS (Figure 5). OCT showed thick nerve fiber layer and GCC with a large disc area of 3.17mm2 OD and 3.05mm2 OS (Figure 6). Visual field was unremarkable OD and OS. The angles were open to ciliary body with grade 1 pigmentation of trabecular meshwork with no synechiae. The patient mentioned her eyes were becoming redder and more irritated. Biomicroscopy revealed grade 1 superficial punctate keratitis and bulbar conjunctival injection.
While she had large cupping, she also had a large disc, and her nerve fiber layer and GCC was unremarkable with excellent symmetry. We explained that she may not need treatment and that it would be reasonable to discontinue. She agreed. One year later, all testing was stable off treatment.
 |
|
Fig. 6. Thick NFL and GCC with large disc OD and OS. Click image to enlarge. |
Balancing Underdiagnosis and Overdiagnosis
Glaucoma, the leading cause of blindness for adults over 60, often progresses silently. While underdiagnosis remains a major concern (estimates suggest up to 78% of cases are missed), recent studies highlight a growing issue: overdiagnosis.28-30
The challenge lies in the complexity of the disease itself. The optic nerve can vary greatly in appearance, and interpreting tests such as OCT requires significant expertise. This complexity was highlighted by Claude Burgoyne, MD, at a previous Optometric Glaucoma Society Meeting, who compared interpreting OCT scans to the work of a radiologist, suggesting a need for subspecialization within the field.
Comorbidities such as retinal artery and vein occlusion, anterior ischemic optic neuropathy, demyelinating disease, neurosarcoid, toxic optic neuropathy, traumatic optic neuropathy, sexually transmitted disease and tumors can all masquerade as glaucoma and must be carefully ruled out.
Adding to the difficulty, factors like family history can be a double-edged sword. While it’s a known risk factor for glaucoma, individuals with a family history were also 8.69-times more likely to be overdiagnosed.29,31 Similarly, cataract surgery emerged as another risk factor for overdiagnosis, especially when combined with family history.29
The legal landscape in optometry further complicates matters. Since most negligence cases involve missed diagnoses, the stakes are high for optometrists.32 This might lead them to err on the side of caution and diagnose glaucoma even with unclear test results.
While OCT is a valuable tool for early detection, its overuse can lead to unnecessary testing and potentially overdiagnosis. This could occur if optometrists routinely include OCT in basic wellness exams for healthy patients who may not require it. Higher amounts of hyperopia and myopia are known to displace the nerve fiber layer bundles nasally and temporally respectively. This may lead practitioners into diagnosing “red disease,” which means that the OCT database flags normal patients. Conversely, OCTs in which the database shows green may lull the practitioner into a false sense of security when the patient has statistically thick nerve fiber layer in the presence of glaucoma. Thus, OCTs are a supplementary tool and should not be viewed in isolation.
In the dynamic landscape of optometric care, assuming responsibility for existing glaucoma patients demands a delicate balance of diligence and empathy. Entering the patient’s journey with a review of past records sets the stage for informed decision-making. Navigating through handwritten records, electronic health records and faxed documents underscores the need for meticulous scrutiny. Understanding the multifactorial nature of glaucoma, from IOP fluctuations to OCT nuances, is paramount. Embracing the challenges of interpreting IOP measurements amidst circadian rhythms and corneal biomechanics, as well as grasping the subtleties of visual field testing, underscores the complexity of the diagnostic process. The pivotal decision-making juncture arises in determining whether to continue, adjust or discontinue treatment.
Takeaways
Through illustrative cases, this article delineates the intricate dance of clinical judgment, balancing the patient’s needs, treatment efficacy, and potential risks. However, this narrative extends beyond individual cases, delving into the broader issue of underdiagnosis and overdiagnosis in glaucoma care. Driven by the recognition of silent progression and the imperative for timely detection, optometrists are challenged to navigate the intricate terrain of diagnostic precision. Acknowledging the dual specters of underdiagnosis and overdiagnosis, glaucoma clinicians must take a nuanced approach, fostering a balance between proactive vigilance and judicious restraint in optometric practice.
Dr. Cymbor is the medical director of the Glaucoma Institute of State College, a member of the Optometric Glaucoma Society and a Lead OD at Nittany Eye Associates in State College, PA. His disclosures for this article include consulting for Visionix and Thea Pharmaceuticals.
Dr. Seitz currently works for University Eye Associates, a private group practice in Charlotte, NC, and has a special interest in managing glaucoma and ocular surface disease. She has no financial disclosures.
1. Zhang N, Wang J, Li Y, Jiang B. Prevalence of primary open angle glaucoma in the last 20 years: a meta-analysis and systematic review. Sci Rep. 2021;11(1):13762. 2. de Venecia G, Davis MD. Diurnal variation of intraocular pressure in the normal eye. Arch Ophthalmol. 1963;69(6):752-7. 3. Stamper RL. A history of intraocular pressure and its measurement. Optom Vis Sci. 2011;88(1):E16-28. 4. Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol. 1993;38(1):1-30. 5. Jiang X, Torres M, Varma R; Los Angeles Latino Eye Study Group. Variation in intraocular pressure and the risk of developing open-angle glaucoma: The Los Angeles Latino Eye Study. Am J Ophthalmol. 2018;188:51-9. 6. Sun L, Chen W, Chen Z, Xiang Y, Guo J, Hu T, Xu Q, Zhang H, Wang J. Dual effect of the Valsalva maneuver on autonomic nervous system activity, intraocular pressure, Schlemm’s canal and iridocorneal angle morphology. BMC Ophthalmol. 2020;20(1):5. 7. Dada T, Gwal RS, Mahalingam K, et al. Effect of “365 breathing technique” on intraocular pressure and autonomic functions in patients with glaucoma: a randomized controlled trial. J Glaucoma. 2024;33(3):149-54. 8. Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44(5):367-408. 9. Susanna CN, Diniz-Filho A, Daga FB, et al. A prospective longitudinal study to investigate corneal hysteresis as a risk factor for predicting development of glaucoma. Am J Ophthalmol. 2018;187:148-52. 10. Medeiros FA, Meira-Freitas D, Lisboa R, et al. Corneal hysteresis as a risk factor for glaucoma progression: a prospective longitudinal study. Ophthalmology. 2013;120(8):1533-40. 11. Turgut B. Pearls for correct assessment of optic disc at glaucoma diagnosis. US Ophthalmic Rev. 2017;10(2):104–10. 12. Iorio-Aranha F, de Freitas C, Rocha-Sousa A, et al. Nationwide consensus on quality indicators to assess glaucoma care: a modified Delphi approach. Eur J Ophthalmol. 2024;34(1):217-25. 13. Hood DC, La Bruna S, Durbin M, et al. Anatomical features can affect OCT measures used for clinical decisions and clinical trial endpoints. Transl Vis Sci Technol. 2024;13(4):27. 14. Harizman N, Oliveira C, Chiang A, et al. The ISNT rule and differentiation of normal from glaucomatous eyes. Arch Ophthalmol. 2006;124(11):1579-83. 15. Sun Young Kim, Hae-Young L. Park, Chan Kee Park. The effects of peripapillary atrophy on the diagnostic ability of Stratus and Cirrus OCT in the analysis of optic nerve head parameters and disc size. Invest Ophthalmol Vis Sci. 2012;53(8):4475-84. 16. Lee J, Kim YK, Ha A, et al. Temporal raphe sign for discrimination of glaucoma from optic neuropathy in eyes with macular ganglion cell-inner plexiform layer thinning. Ophthalmology. 2019;126(8):1131-9. 17. Koç A, Özcura F, Gültekin Irgat S, Arik Ö. Evaluation of retinal layers in individuals with pseudoexfoliation syndrome and ocular hypertension. J Glaucoma. 2024;33(5):325-33. 18. Jung KI, Kim SJ, Park CK. Systemic vascular risk factors for multiple retinal nerve fiber layer defects. Sci Rep. 2018;8(1):7797. 19. Brandao LM, Ledolter AA, Schötzau A, Palmowski-Wolfe AM. Comparison of two different OCT systems: retina layer segmentation and impact on structure-function analysis in glaucoma. J Ophthalmol. 2016;2016:8307639. 20. Baig S, Diniz-Filho A, Wu Z, et al. Association of fast visual field loss with risk of falling in patients with glaucoma. JAMA Ophthalmol. 2016;134(8):880-6. 21. Asrani S. Screening and establishing a diagnosis with OCT. Glaucoma Today. March/April 2024. glaucomatoday.com/articles/2024-mar-apr/screening-and-establishing-a-diagnosis-with-oct. Accessed May 17, 2024. 22. Prum BE Jr, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma – preferred practice pattern. Ophthalmology. 2016;123(1):P41–111. 23. Bradley C, Hou K, Herbert P, et al. Evidence-based guidelines for the number of peripapillary OCT scans needed to detect glaucoma worsening. Ophthalmology. 2023;130(1):39-47. 24. Kitazawa Y, Yamamoto T. Glaucomatous visual field defects: their characteristics and how to detect them. Clin Neurosci. 1997;4(5):279-83. 25. Phu J, Kalloniatis M. Viability of performing multiple 24-2 visual field examinations at the same clinical visit: the Frontloading Fields Study (FFS). Am J Ophthalmol. 2021;230:48-59. 26. Leung C, Garway-Heath DF, Medeiros FA, et al, eds.WGA Consensus Series 8. Progression of Glaucoma. Kugler Publications; 2011. 27. Stagg BC, Stein JD, Medeiros FA, et al. The frequency of visual field testing in a US nationwide cohort of individuals with open-angle glaucoma. Glaucoma. 2022;5(6):587-93. 28. Shaikh Y, Yu F, Coleman AL. Burden of undetected and untreated glaucoma in the United States. Am J Ophthalmol. 2014;158(6):1121-9.e1. 29. Founti P, Coleman AL, Wilson MR, et al. Overdiagnosis of open-angle glaucoma in the general population: the Thessaloniki Eye Study. Acta Ophthalmol. 2018;96(7):e859-64. 30. Lin M, Xiao Y, Hou B, et al. Evaluate underdiagnosis and overdiagnosis bias of deep learning model on primary open-angle glaucoma diagnosis in under-served populations. AMIA Jt Summits Transl Sci Proc. 2023;2023:370-7. 31. Le A, Mukesh BN, McCarty CA, Taylor HR. Risk factors associated with the incidence of open-angle glaucoma: the visual impairment project. Invest Ophthalmol Vis Sci. 2003;44(9):3783-9. 32. Duszak RS, Duszak R Jr. Malpractice payments by optometrists: an analysis of the national practitioner databank over 18 years. Optometry. 2011;82(1):32-7. |

