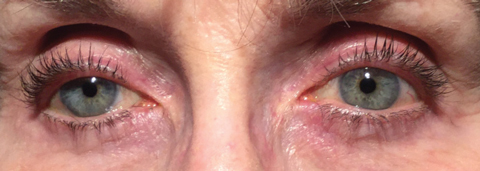
An estimated 2.2 million Americans have glaucoma and 20 million have dry eye disease (DED)—odds are, practitioners are bound to see patients diagnosed with both.1,2 Research suggests the comorbidity of DED in patients treated topically for ocular hypertension and glaucoma could be as high as 20% to 59%.3 But few step back to consider the association between these two chronic and progressive diseases.
Often, it’s nearly impossible to decipher which disease came first and how much of the DED is iatrogenic—caused inadvertently by a medical treatment or procedure.
DED may stem from the medical treatment of glaucoma, for example. Studies show 38% of glaucoma patients are using a tear substitute, and the mainstay topical medications for glaucoma management come with side effects such as allergy, toxicity, immuno-inflammatory effects, punctate keratitis, conjunctival inflammation and disruption of the tear film.4,5 These all result in reduction of the lipid and aqueous layers, damage to the goblet cells and neurotoxicity to corneal nerves.4,5 With each additional medication involved in the treatment of glaucoma, the risk of an adverse event or possible exacerbation of dry eye multiplies.6-8 Here’s a look at the ocular surface in patients being treated for glaucoma—and how you can help protect it.
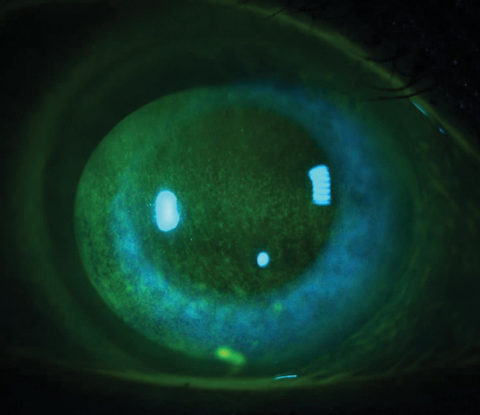 |
| Fig. 1. Corneal staining in the form of punctate epitheliopathy is a diagnostic finding for DED. Many BAK-containing glaucoma medications can cause this presentation, so ask glaucoma patients about ocular comfort during follow-up, as duration of use and multiple medication use increases the risk. Photo: Jacob R. Lang, OD |
Treatment-related DED
Topical intraocular pressure (IOP)-lowering medication is often the first-line treatment for glaucoma patients. These agents not only contain their active ingredients for IOP lowering, but also excipients, including buffers, preservatives, drug vehicle and viscosity agents—all of which may negatively impact the ocular surface (Table 1). Some risk factors for iatrogenic DED as a result of topical glaucoma treatment include the duration of treatment, concentration of preservatives in the medication, the number of medications being used, a higher baseline IOP and disease severity.6,9,10
While randomized clinical trials of contemporary glaucoma drugs show decent tolerability, studies fail to depict their effects on the ocular surface because patients at risk for ocular surface disease are excluded, the study durations are short and they don’t evaluate the effects of multiple meds on the ocular surface.5
Researchers now know the negative effects of glaucoma medications on the ocular surface are dose-dependent.7,8,11 In addition, studies show the prevalence of DED increases with multiple medication use.6-8 In one study, 11% of patients using one medication reported dry eye symptoms, while 39% on two medications and more than 43% on three medications reported symptoms such as irritation, foreign body sensation, transiently blurred vision and increased blinking.12
Table 1. Known Ocular Side Effects From Glaucoma Medications | |
| Medication | Ocular Side Effects |
| Carbonic anhydrase inhibitor | Allergic reaction, blepharitis, blurred vision, burning/stinging, conjunctival edema, conjunctivitis, discharge, dryness, hyperemia, keratitis, photophobia, tearing |
| Alpha-agonist | Burning/stinging, blepharitis, blurred vision, conjunctival blanching, conjunctival follicles, conjunctival hyperemia, conjunctivitis, dry eye, keratitis, eyelid erythema, foreign body sensation, lid edema, pain, photophobia, tearing |
| Beta-blocker | Burning/stinging, blurred vision, conjunctivitis, contact dermatitis, eyelid erythema, photophobia, punctate keratitis |
| Prostaglandin analogs | Allergic reaction, burning/stinging, blepharitits, blurred vision, cataract, conjunctivitis, cystoid macular edema, dry eye, eyelash growth, eyelid skin darkening, foreign body sensation, hyperemia, iris color changes, iritis, pain, punctate keratitis |
| Cholinergic agents | Burning/stinging, blurred vision, conjunctival injection, decreased night vision, decreased vision due to ciliary spasm, myopia and retinal detatchment (rare), keratitis, periorbital headache (brow ache), tearing |
The very pharmacokinetics of glaucoma medications can cause tear film alterations.5 Medication administration onto the ocular surface for 15 to 20 seconds provides a low bioavailability of less than 5%, and absorption is affected by the medication’s pH level and viscosity.11,13 Absorption is also influenced by the drug’s tear solubility and ocular surface permeability. For example, prostaglandin analogs (PGAs) require corneal esterases to convert the drug to the free acid form, which has the actual therapeutic effect as a prodrug.14 The retention time is longer for more viscous solutions, allowing for increased absorption. This influences the drug’s bioavailability and thus efficacy, but also increases the risk of an adverse effects (Figure 1).
The pH of a medication is also important for corneal penetration and absorption of the therapeutic agent. A healthy tear film has a pH ranging from 7.3 to 7.7 and is at the lowest (more acidic) in the morning upon awakening.15 For a glaucoma medication to be comfortable with instillation, the pH should be between 6 and 8.15 A topical glaucoma medication within this range allows for better drug absorption due to decreased lacrimation from discomfort, as well as less risk for tissue damage.
Some drugs, such as Trusopt (dorzolamide, Merck), Cosopt (dorzolamide hydrochloride-timolol maleate, Akorn) and Zioptan (tafluprost ophthalmic solution, Akorn) have a lower pH at 5.6, 5.65 and 5.5, respectively, making them less tolerable with increase symptoms of instillation irritation and lacrimation with instillation (Table 2).
Many glaucoma drugs contain preservatives, namely benzalkonium chloride (BAK). This quaternium ammonia is necessary to prevent microbial contamination and prevent breakdown of the active ingredient in multidose formulations. While good for bioavailability of the medication, they are not ideal for corneal health. BAK, for example, disrupts tear film homeostasis by stripping away the lipid layer, leading to increased evaporation and entry into the vicious circle of tear film instability.15 Once in the vicious circle, hyperosmolarity of the tear film is followed by inflammation to the ocular surface, release of an inflammatory cascade of cytokines and damage to goblet cells and corneal and conjunctival tissues; if left untreated, the cycle perpetutes.16
One survey of 9,658 glaucoma patients found a significant difference in patient experience between preservative-free and preserved medications.17 Eliminating preservatives led to a decrease in dry eye sensations from 34.9% to 16%, and simply by removing BAK, the clinical finding of corneal staining, a diagnostic criteria for DED, was reduced by 35%.16 Another study found switching to preservative-free formulations reduced discomfort upon instillation, foreign body sensation, dry eye symptoms, tearing and eyelid itching.18 The newer PGAs with preservative-free formulations and alternative preservatives show similar improvement of symptoms.19 Researchers also found ocular surface improvement occurred as early as one month after removing the offending agent.20
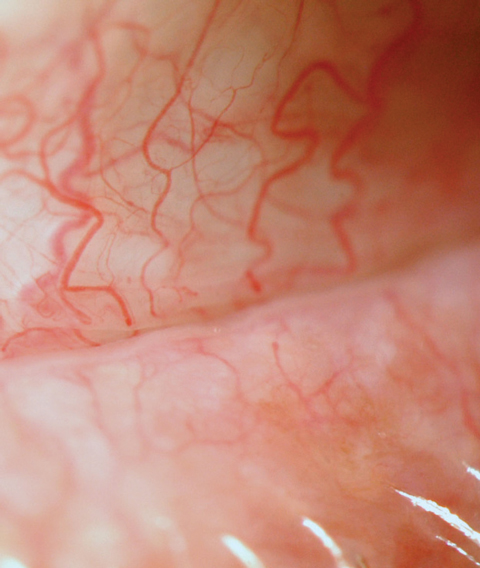 |
| Fig. 2. Inflammation is present in all forms of DED. Conjunctival hyperemia to the meibomian gland orifice can be seen clinically, as pictured here. |
Stay Proactive: Evaluate
When examining a patient using at least one glaucoma drug, clinicians should always pay close attention to the ocular surface, ask about dry eye symptoms and evaluate for signs of DED. The five-item Dry Eye Questionnaire (DEQ-5) or the Ocular Surface Disease Index (OSDI) should be repeated at every patient encounter, as the effects of topical glaucoma medications can be cumulative. DED is suspected if DEQ-5 is greater than six and if the OSDI staging of severity is mild (13 to 22), moderate (23 to 32) or severe (greater than 32). To evaluate for clinical signs of DED, clinicians can use one of three TFOS DEWS II recommended tools: noninvasive tear break-up time (NTBUT) or TBUT with fluorescein dye; tear film osmolarity; or corneal, conjunctival and lid staining with fluorescein and lissamine green vital dyes.21 The dry eye testing sequence is important and should be performed from least to most invasive.
Osmolarity provides a measure of the tear chemistry including tear film stability and homeostasis. TearLab’s osmolarity system collects and analyzes a 50nL sample of tears obtained from the inferior lateral meniscus and lid margin. Normal osmolarity ranges from 290mOsms/L to 300mOsms/L. An osmolarity of 309mOSms/L to 328mOSms/L is categorized as mild to moderate, and higher than 328mOsms/L is considered severe.22 An inter-eye difference of 8mOsms/L or greater also indicates tear film instability and may be more diagnostic of DED than the level of osmolarity in each eye.23
NTBUT measures, as well as TBUT with fluorescein dye, should always be taken after tear osmolarity. A TBUT reading of 10 or less indicates DED.21
Another point-of-care test currently available in the United States includes the InflammaDry test (Quidel) for the detection of elevated levels of matrix metalloproteinase (MMP-9) in tears (Figure 2).
Table 2. Glaucoma Medication pH Values | |
| Medication | pH Value |
| Beta-blockers | |
| Betagan (levobunolol 0.25%, 0.5%, Allergan) | 5.5 to 7.5 |
| Betimol (timolol hemihydrate 0.25%, 0.5%, Santen) | 6.5 to 7.5 |
| Betoptic S (betaxolol 0.5%, Alcon) | 7.6 |
| Carteolol 1.0% | 6.2 to 7.2 |
| Istalol (timolol maleate 0.5%, Bausch + Lomb) | 6.5 to 7.5 |
| Timoptic, Timoptic XE (gel) (timolol maleate 0.25%, 0.5%, Bausch + Lomb) | 7.0 |
| Prostaglandin Analogs | |
| BAK-free latanoprost | 7.0 |
| Lumigan (bimatoprost, Allergan) | 6.8 to 7.8 |
| Travatan Z (travoprost, Alcon) | 5.7 |
| Xalatan (latanoprost, Pfizer) | 6.7 |
| Zioptan (talfluprost, Akorn) | 5.5 to 6.7 |
| Alpha-agonist | |
| Alphagan P (brimonidine 0.1%, Allergan) | 7.4 to 8.0 |
| Alphagan P (brimonidine 0.15%, Allergan) | 6.6 to 7.4 |
| Alphagan (brimonidine 0.2%, Allergan) | 5.6 to 6.6 |
| Carbonic Anhydrase Inhibitors | |
| Azopt (brinzolamide, Alcon) | 7.5 |
| Trusopt (dorzolamide, Merck) | 5.6 |
| Combination Therapy | |
| Combigan (brimolidine/timolol, Allergan) | 6.5 to 7.3 |
| Cosopt (dorzolamide/timolol, Akorn) | 5.65 |
| Simbrinza (brinzolamide/brimonidine, Alcon) | 6.5 |
Protect the Ocular Surface
When left undetected or untreated, DED can significantly impact glaucoma management, as increased symptoms of ocular discomfort can lead to decreased medication compliance—a known risk factor for disease progression.23
In addition, tear film disruption can affect the reliability and reproducibility of diagnostic testing such as visual fields and OCT. One study found artificial tears QID for one week prior to visual field testing for patients with DED and glaucoma improved test time and results.24
When it comes to surgical management of glaucoma, a healthy ocular surface is even more important. Conjunctival inflammation from prolonged exposure to BAK can result in poor surgical outcomes due to conjunctival scarring.25 Research also shows an association between long-term topical glaucoma medication use and fibroblast proliferation of Tenon’s capsule and elevated MMP-9 levels, which can contribute to filtering bleb scarring following trabeculectomy.26 Another study shows inferior fornix shortening with topical glaucoma therapy for three or more years.26
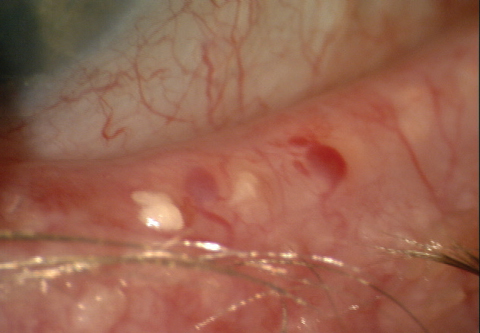 |
| Fig. 3. MGD is a common cause of evaporative dry eye. Examining the lid for structural and functional gland changes—such as thickened meibomian gland secretions, as seen here—should be routine. |
The Meibomian Glands
While the many adverse events to the tear film and ocular surface associated with topical glaucoma treatments are well known, the impact on the meibomian glands is not. One study looked at the effects of beta-blockers and PGAs on meibomian gland function and morphology and found a positive correlation between topical treatments and decreased gland structure and function.27
Another study of meibomian gland epithelial cell survival rates when exposed to pilocarpine and timolol found a dose-dependent survival rate on cell culture, but not with the drug levels accumulating near the glands during instillation.28
Much is unknown about the meibomain glands and their function, but clinical practice suggests PGAs have an inflammatory effect on the meibomian glands, evidenced by the thickened and red eyelid margin and tissue surrounding the meibomian glands (Figure 3). Meibomian gland assessment should remain an integral part of the comprehensive dry eye exam for glaucoma patients.
Table 3. BAK Concentrations in Glaucoma Medications | |
| Medication | BAK (%) |
| Alphagan P (brimonidine, Allergan) | BAK-free |
| Cosopt PF (dorzolamide/timolol, Akorn) | BAK-free |
| Timoptic XE (timolol maleate, Bausch + Lomb) | BAK-free |
| Travatan Z (travoprost, Alcon) | BAK-free |
| Zioptan (talfluprost, Akorn) | BAK-free |
| Simbrinza (brinzolamide/brimonidine, Alcon) | 0.003 |
| Alphagan (brimonidine, Allergan) | 0.005 |
| Betagan (levobunolol, Allergan) | 0.005 |
| Combigan (brimolidine/timolol, Allergan) | 0.005 |
| Lumigan (bimatoprost, Allergan) | 0.005 |
| Cosopt (dorzolamide/timolol, Akorn) | 0.0075 |
| Trusopt (dorzolamide, Merck) | 0.0075 |
| Azopt (brinzolamide, Alcon) | 0.01 |
| Betoptic S (betaxolol, Alcon) | 0.01 |
| Timoptic (timolol maleate, Bausch + Lomb) | 0.01 |
| Xalatan (latanoprost, Pfizer) | 0.02 |
Treatment: Choose Wisely
Many choices exist when managing glaucoma patients, and clinicians should try to reduce the risk of iatrogenic DED whenever possible, especially in patients with multiple factors associated with dry eye. PGAs are often the first-line choice, as they offer great IOP reduction and convenient dosing. However, PGAs can lead to meibomian gland obstruction, which makes it less than ideal for patients with pre-existing MGD. Topical beta-blockers, however, can reduce aqueous secretions and may not be ideal for patients with autoimmune conditions such as Sjögren’s syndrome, where aqueous secretions are already reduced.
A number of preservative-free and BAK-free glaucoma medications exist. Clinicians should consider starting patients with a BAK-free medication if possible. If a medication with BAK is unavoidable, a medication with the lowest concentration is the best option to start with. Before adding a second medication, clinicians should consider changing to a combination therapy to reduce the BAK load on the ocular surface, Cosopt PF is the only combination medication offering combination therapy with a BAK-free formulation (Table 3). ImprimisRx, a new concept in the pharmaceutical industry, uses compounding pharmacies to formulate preservative-free medication options (Table 4). While promising, these drugs should be used with caution, as they do not undergo the same FDA screening for safety, and contamination is a concern.
Alternative therapies such as selective laser trabeculoplasty (SLT) or minimally invasive glaucoma surgery (MIGS) may eliminate medication-induced effects, which should reduce the risk for dry eye symptoms. Research shows SLT is effective for both primary and adjunctive glaucoma treatment with a mean IOP reduction of 3.8mm Hg to 8.0mm Hg after six months to one year and a mean success rate of 55% to 82 % in the same time frame.29 MIGS surgery can also be effective at lowering IOP, and lowers patients’ dependency on topical therapies by increasing outflow through Schlemm’s canal.30
Table 4. ImprimisRx Glaucoma Combination Drop Therapy | ||
| Medication | Concentration | Unit |
| LAT (latanoprost) | 0.005% | 5mL |
| TIM-LAT (timolol/latanoprost) | 0.5/0.005% | 5mL |
| BRIM-DOR (brimonidine/dorzolamide) | 0.15/2% | 10mL |
| TIM-BRIM-DOR (timolol/brimonidine/dorzolamide) | 0.5/0.15/2% | 10mL |
| TIM-DOR-LAT (timolol/dorzolamide/latanoprost) | 0.5/2/0.005% | 5mL |
| TIM-BRIM-DOR-LAT (timolol/brimonidine/dorzolamide/latanoprost) | 0.5/0.15/2/0.005% | 5mL |
| Triple/quad kit: •TIM-BRIM-DOR (timolol/brimonidine/dorzolamide) •TIM-BRIM-DOR-LAT (timolol/brimonidine/dorzolamide/latanoprost) | 0.5/0.15/2% 0.5/0.15/2/0.005% | 10mL 5mL |
| ImprimisRx. Compounded ophthalmic formulations. September 2017. www.imprimisrx.com/assets/IMPO0114-Rev6_Foldout-Product-List_pgs.pdf. Accessed October 4, 2017. | ||
Restore the Tear Film
Despite rigorous follow up and scrutiny, glaucoma patients often end up with iatrogenic DED from topical therapy. When treating the ocular surface, the main focus should be on restoring homeostasis and reducing the inflammatory response to the topical medications. Therapies such as Restasis (cyclosporine, Allergan) or Xiidra (lifitegrast, Shire) are often the first choice therapy for DED. As these add to a patient’s daily list of drops, proper education is paramount to ensure they wait between drop for proper clearance of each medication.
Patient education on the side effects of DED therapy, including burning with instillation, blurred vision and dysgeusia, will improve medication compliance rates. It is possible that as the ocular surface heals, some of the side effects could diminish. Treatments for MGD include heat with at-home heat masks, as well as in-office LipiFlow (TearScience) and manual expression—an often necessary step, though it adds to your patient’s treatment burden.
Pitfalls of PGA TreatmentGlaucoma patients using PGAs may be at risk for prostaglandin-associated periorbitopathy, a condition that includes: • Ptosis Many of these side effects are irreversible, and clinicians should be cautious when initiating treatment with PGA medications, especially for patients treated monocularly. Patients should also be educated on the possible cosmetic side effects. |
As with all patients, follow up is key. Patients with DED and glaucoma are often juggling multiple medications, and return visits are important to continue to monitor the ocular surface to ensure both treatments remain effective.
Identifying dry eye in patients undergoing glaucoma treatment is integral to comprehensive care. Often, we are so busy attending to the first disease that we ignore the fact that its remedy may adversely affect the ocular surface. But identifying DED in our glaucoma patient population may allow for earlier intervention, including a chance in glaucoma management and initiation of DED therapies.
Dr. O’Dell is the director of the Dry Eye Center of Pennsylvania at Wheatlyn Eye Care in Manchester, Pa.
Dr. Gaddie is owner and director of Gaddie Eye Centers in Louisville, Ky.
1. Friedman DS, Wolfs RC, O’Colmain BJ; Eye Diseases Prevalence Research Group. Prevalence of open-angle glaucoma in the United States. Arch Ophthalmol. 2004;122:532-8. |