Polish Your Dx SkillsNo matter how long you've been in practice, it's always worthwhile to brush up on your diagnostic techniques and incorporate newer ones when you can. This month in Review of Optometry, experts review how to refine your slit-lamp skills, distinguish between optic nerve differentials, use head-mounted perimetry and pay closer attention to detail in peripheral fundus photos. Check out the other articles featured in the February 2024 issue: |
Acute vision loss is enough to set off alarms in the office, but add disc swelling to the presentation and the case quickly becomes an ocular emergency that needs to be tended to right away. It is important to thoroughly test these patients to make the appropriate diagnosis and come up with a proper management plan. During a busy clinic day, this is easier said than done, so developing a systematic approach to disc edema helps efficiently narrow down the differentials. Adapting this protocol can ultimately save a patient’s vision, and in some cases, a patient’s life.
There are a few definitions of disc swelling to outline before categorizing the differential diagnoses:
• Pseudopapilledema: appearance of swollen nerves in the absence of true edema.
• Papilledema: bilateral optic disc edema secondary to increased intracranial pressure (ICP).1
• Disc edema: swelling of the optic disc due to fluid accumulation within or around the axons.
 |
|
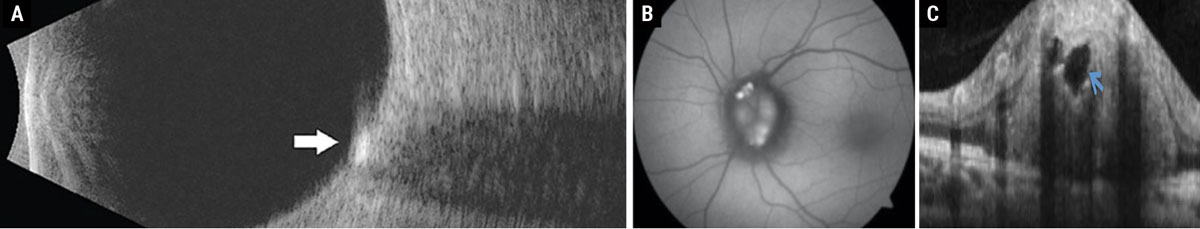
Fig. 1. (a) Disc drusen seen as hyperreflective deposit on B-scan ultrasonography. (b) Disc drusen exhibiting hyperautofluorescence on FAF photography. (c) Disc drusen seen as a signal-poor core with hyperreflective borders on enhanced-depth imaging OCT. Click image to enlarge. |
Pseudopapilledema
These cases are easiest to diagnose with fundus photography and OCT imaging. Patients are typically asymptomatic and the nerves usually arouse suspicion during the dilated fundus exam or on routine fundus photography. Differential diagnoses for pseudopapilledema include disc drusen, congenitally anomalous nerves, vitreopapillary traction and a peripapillary choroidal neovascular membrane.
Disc drusen are acellular deposits of proteins and calcium that have an autosomal dominant inheritance pattern.2 Eighty-seven percent of these patients, particularly those with superficial disc drusen, will develop visual field defects that can manifest from an enlarged blind spot to a nasal or arcuate defect.2,3 Although hyperreflectivity on B-scan ultrasonography used to be the gold standard for diagnosis, the prevalence and ease of OCT instruments has made enhanced depth imaging the diagnostic test of choice in recent times.3 Superficial disc drusen will hyperautofluoresce on fundus autofluorescence (FAF) and stain with fluorescein angiography (Figure 1).3 Patients with disc drusen should be monitored with serial perimetry and optic nerve scans annually. Although there is no standard of care for treatment in patients with structural or functional progression, topical brimonidine may be considered off-label due to its possible neuroprotective benefits.2,3
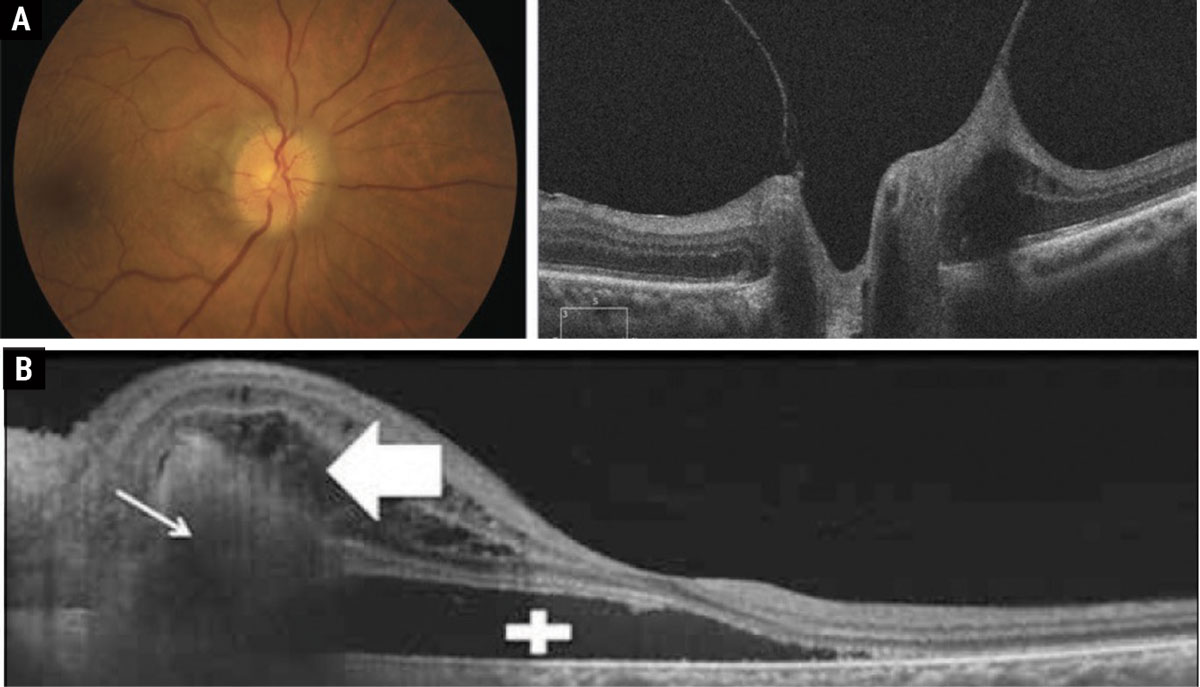
A hallmark sign to look for in patients with congenitally anomalous nerves is the presence of a spontaneous venous pulse (SVP), which is present in 80% of the normal population.4 However, it is important to note that ICP, like intraocular pressure, exhibits diurnal fluctuation. So, although an SVP is present during the exam, it may not be enough to rule out papilledema in the presence of other symptoms. Other physiologic changes within the nerve that can cause the appearance of indistinct nerve margins are vitreopapillary traction and peripapillary choroidal neovascular membranes, both of which can be identified with OCT (Figure 2).
 |
|
Fig. 2. (a) Vitreopapillary traction causing the appearance of pseudopapilledema. (b) Peripapillary choroidal neovascular membranes with subretinal fluid causing the appearance of elevated disc margins. Click table to enlarge. |
Papilledema
Causes of ICP include intracranial masses, increased venous pressure, inflammation, sleep apnea and medications. Although idiopathic intracranial hypertension (IIH) is the most common cause, it is a diagnosis of exclusion.1,5
Patient History
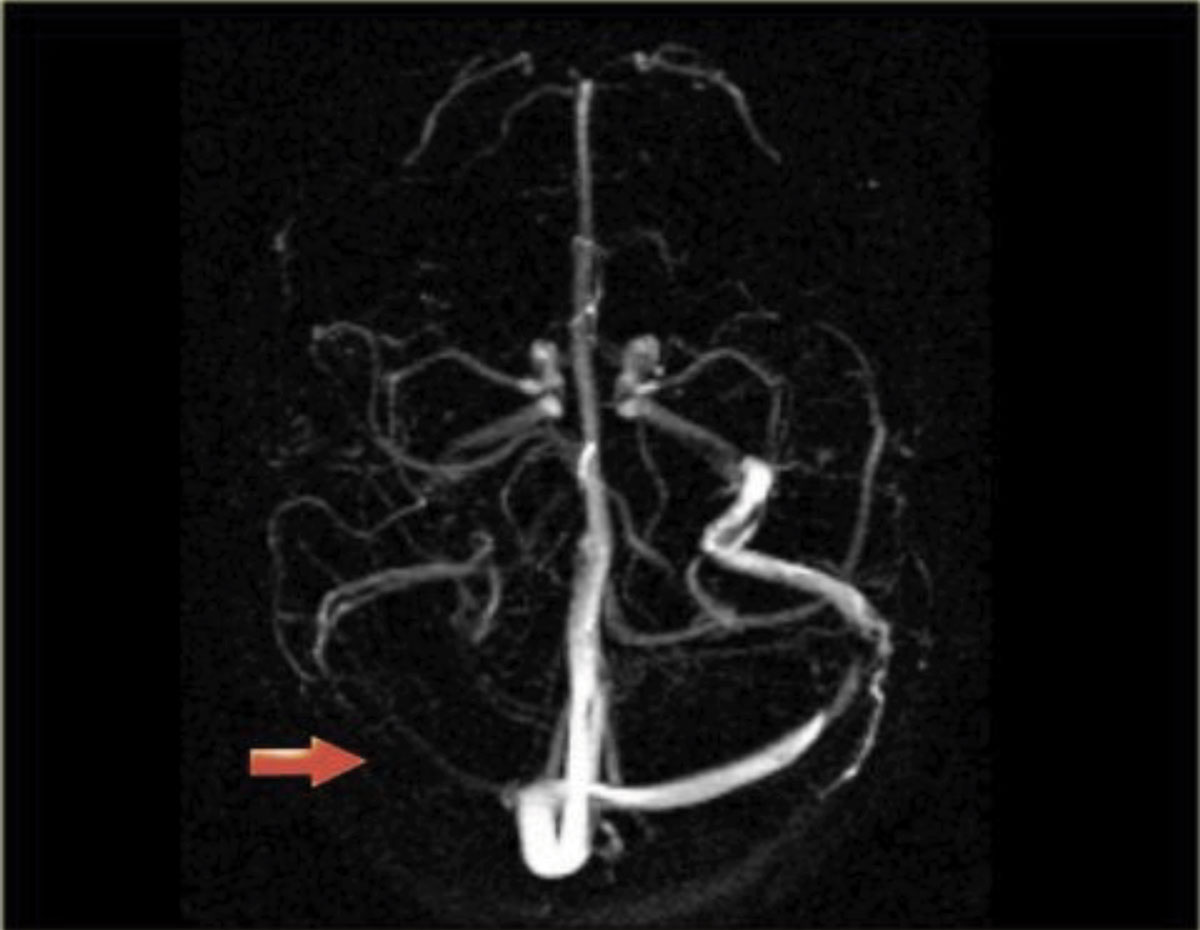
The patient’s presenting symptoms and history can help narrow down the differential diagnoses for papilledema. The typical clinical profile for an IIH patient is an overweight female of childbearing age. Beyond that, increased intracranial pressure most commonly causes headaches (worse when supine), pulsatile tinnitus, transient visual obscurations with postural changes, and diplopia.1,5 Recent history of a fall or head trauma can indicate the presence of an intracranial hemorrhage. The existing diagnosis of a hypercoagulability disorder in the background of a persistent headache may raise suspicion for cerebral venous sinus thrombosis (Figure 3).6
Certain medications can cause papilledema as well, so it is important to rule out the current or recent use of the following substances: tetracyclines, hormonal contraceptives, ethambutol, amiodarone, isoniazid, vitamin A derivatives (Accutane, tretinoin), lithium, sex hormones and steroids.7 Lead and methanol poisoning can also cause papilledema.7
 |
|
Fig. 3. Magnetic resonance venography showing that the right venous sinus has no signal due to thrombosis. Click image to enlarge. |
Entrance Testing
Here is what to look for when examining patients with papilledema:
• Visual acuity: usually unaffected unless the patient has advanced nerve atrophy or visual field loss.
• Blood pressure: within normal limits.
• Pupils: (-)APD.
• EOMs: (+)sixth nerve palsy (unilateral or bilateral), third and fourth nerve palsies less common.
• Visual field: enlarged blind spot is most common, nasal step is the second most common defect.
Imaging
Use available imaging tools to narrow down the differentials and document disease progression:
• Fundus photography. Photo documentation of baseline nerve appearance is very useful for patient education, follow-ups and comanagement.
• OCT nerve and ganglion cell complex. Initial scans will show thickening of the retinal nerve fiber layer to some extent, but it is important to get baseline nerve scans on all papilledema (and pseudopapilledema) patients to monitor for change over time. The goal is to avoid irreversible optic atrophy with timely referral and treatment, but prognosis can be difficult to judge in the early stages.
• MRI of brain and orbits with contrast/MRV. Head imaging is key in identifying other causes of elevated ICP, such as masses, hydrocephalus, clots, cerebral inflammatory disorders or venous insufficiencies. Lumbar punctures induce an acute pressure gradient that can cause brain herniation if there are undetected space occupying lesions. This is why it is imperative for every papilledema patient to undergo head imaging prior to proceeding with the next steps.
Management
There are a few considerations to make when managing papilledema:
• Lumbar puncture with cerebrospinal fluid (CSF) analysis. The normal opening pressure for a lumbar puncture is 6cm H2O to 25cm H2O in adults—anything above that indicates elevated ICP.1,5 CSF analysis helps rule out inflammatory, infectious and neoplastic causes of elevated ICP.
• Treatment. Standard for IIH is oral acetazolamide, starting at 500mg BID and titrated as high as 4g BID.1,5 Patients on oral acetazolamide long-term need to get routine renal function testing. In addition, heavy emphasis should be placed on diet and lifestyle, as studies found losing 6% of the body weight yields improvement in signs and symptoms of IIH.1,5
• Sleep study. A diagnosis of sleep apnea should be considered in all papilledema patients. Sleep apnea is thought to cause intermittent increases in ICP as nocturnal hypoxia triggers vasodilation, which has been associated with cases of persistent papilledema that resolved with CPAP use.8 This is a tricky diagnosis because daytime lumbar punctures will be normal in these patients and their symptoms more closely follow silent optic atrophy.
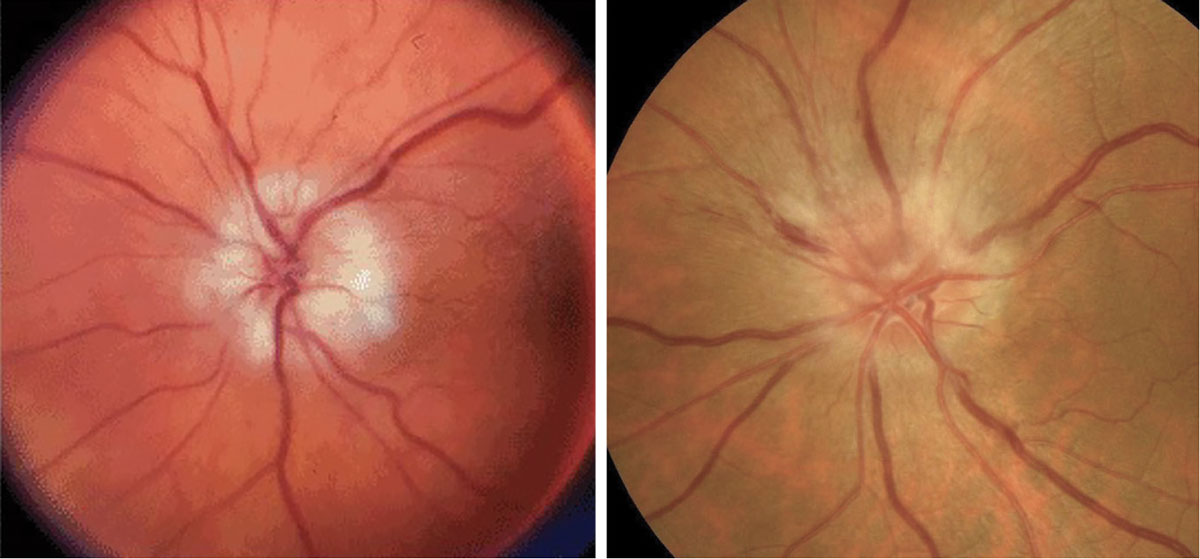 |
Fig. 4. AAION on the left and NAION on the right. Click table to enlarge. |
Disc Edema
Causes of disc edema with normal intracranial pressure can be categorized as ischemic, inflammatory, compressive, infiltrative or hereditary. Non-perfusion leads to failure of ATP-dependent ion transport, the buildup of intracellular sodium and the eventual accumulation of water to maintain the osmotic gradient.9 These physiologic changes cause the appearance of disc edema in cases of arteritic anterior ischemic optic neuropathy (AAION), non-arteritic ischemic optic neuropathy (NAION), central retinal vein occlusions, diabetic papillopathy and malignant hypertension.9-12
Ischemic
These typically present with painless, acute vision loss. It is imperative to rule out AAION in all patients over age 50 due to its high correlation with giant cell arteritis, which can be fatal. In comparison to the hyperemic disc seen in NAION, AAION has a chalkier disc appearance and is often associated with scalp pain, headaches, jaw claudication, fever, amaurosis fugax and weight loss (Figure 4).12,13 NAION has a slightly younger at-risk population (under 50 years old) and occurs secondary to vasculopathic causes, such as hypertension, hyperlipidemia, diabetes and sleep apnea.13 The use of erectile dysfunction medications also increases the risk for NAION. Patients with inherently smaller C/D ratios present with higher risk for NAION, or a “disc at risk.” Both conditions may present with altitudinal field defects and a relative afferent pupillary defect (RAPD). An ischemic central retinal vein occlusion can also present with a RAPD and disc edema, the latter of which resolves with time (Figure 5).11
Differentials of ischemic disc edema that typically do not present with an RAPD include diabetic papillopathy and malignant hypertension. Diabetic papillopathy is a diagnosis of exclusion that resolves with improved blood sugar control and has good visual prognosis.14 Blood pressure readings >180/120 should raise alarms for malignant hypertension, or grade 4 hypertensive retinopathy (Figure 6). These patients need to be sent to the emergency room for immediate blood pressure lowering.
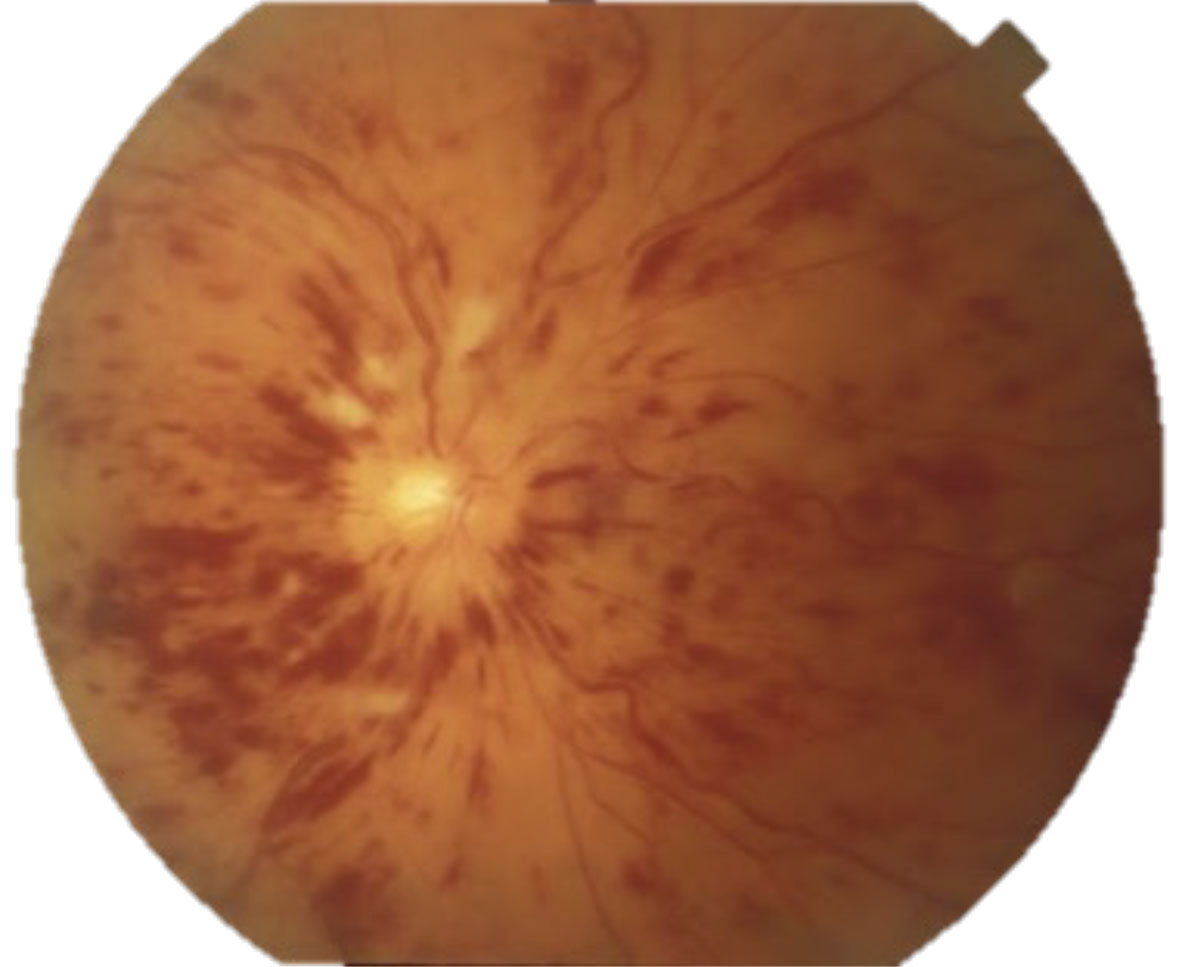 |
|
Fig. 5. Central retinal vein occlusion with disc edema. Click image to enlarge. |
Inflammatory
Optic neuritis should be ruled out in young patients (15 to 45 years) presenting with unilateral, acute vision loss. Other associated symptoms include decreased color vision, (+)RAPD and pain on eye movements. It is important to note that only one-third of optic neuritis cases present with disc edema, while two-thirds of cases are retrobulbar. A lesser-known cause of orbital inflammation is optic perineuritis, which presents with signs and symptoms similar to optic neuritis, but with slower onset and milder effect on central vision.15 The best way to differentiate the two is with directed orbital imaging, in which optic neuritis presents with optic nerve enhancement and possible white matter lesions, while optic perineuritis presents with enhancement around the optic nerve sheath (Figure 7).15
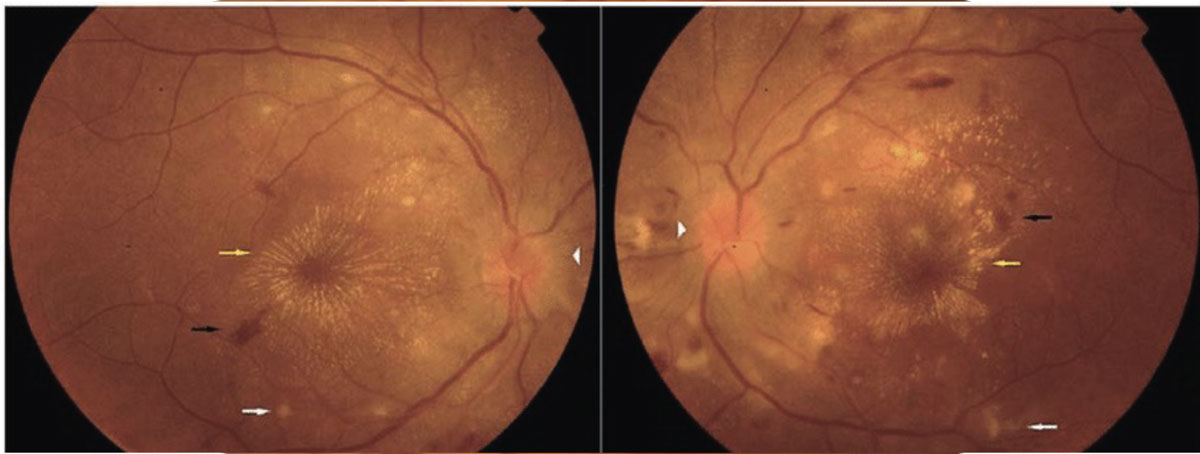 |
|
Fig. 6. Malignant hypertension with bilateral disc edema, macular stars, flame hemes and cotton wool spots. Click image to enlarge. |
Infections
Neuroretinitis is another cause of disc edema with infectious etiology most commonly caused by Bartonella heneslae or quintana, or cat-scratch disease. This condition also presents with unilateral, acute vision loss, dyschromatopsia and a (+)RAPD. Similar to malignant hypertension, it also presents with a macular star. Neuroimaging is normal and not indicated in these cases and treatment can be started empirically while waiting on lab testing.16 Less common causes of infectious disc edema include Lyme disease, syphilis, toxoplasmosis, histoplasmosis and tuberculosis.
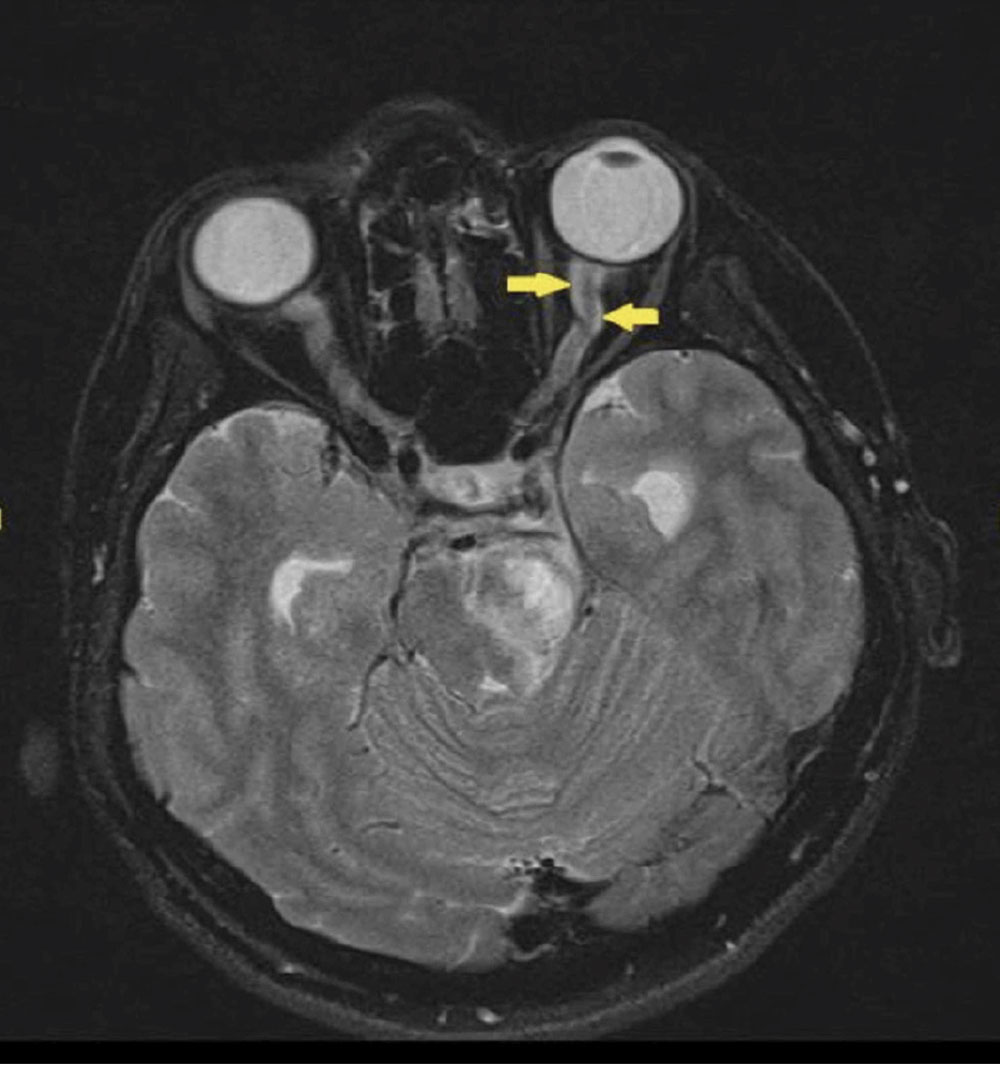 |
|
Fig. 7. Optic nerve sheath enhanced in a case of optic perineuritis, also known as the “tram-track” sign. This sign is not specific to optic perineuritis and can also be seen in optic nerve sheath meningiomas. Click image to enlarge. |
Compressive
Disc edema can manifest in response to compressive forces within the orbit by thyroid orbitopathy, idiopathic orbital inflammation, optic nerve sheath meningioma and other orbital tumors.17 These patients will present with progressive vision loss, dyschromatopsia, proptosis and abnormal extraocular motility. They are best diagnosed with an MRI of the orbits, chiasm and brain with contrast. In thyroid orbitopathy, the MRI will show enlarged recti with tendons spared. This is in contrast to idiopathic orbital inflammation, which is typically unilateral and shows enlargement of tendons, recti and the lacrimal gland. Optic nerve sheath meningiomas will present similarly to optic perineuritis on head imaging, but without the acute symptoms.17
Infiltrative
These tend to have acute explosive presentation secondary to leukemia, lymphoma, metastases from other tumors and sarcoidosis (Figure 8).18 Patients usually have a history of known malignancies and may be symptomatic for progressive vision loss, (+)RAPD and dyschromatopsia.18 Due to the unpredictable nature of the etiology, symptoms can vary widely.
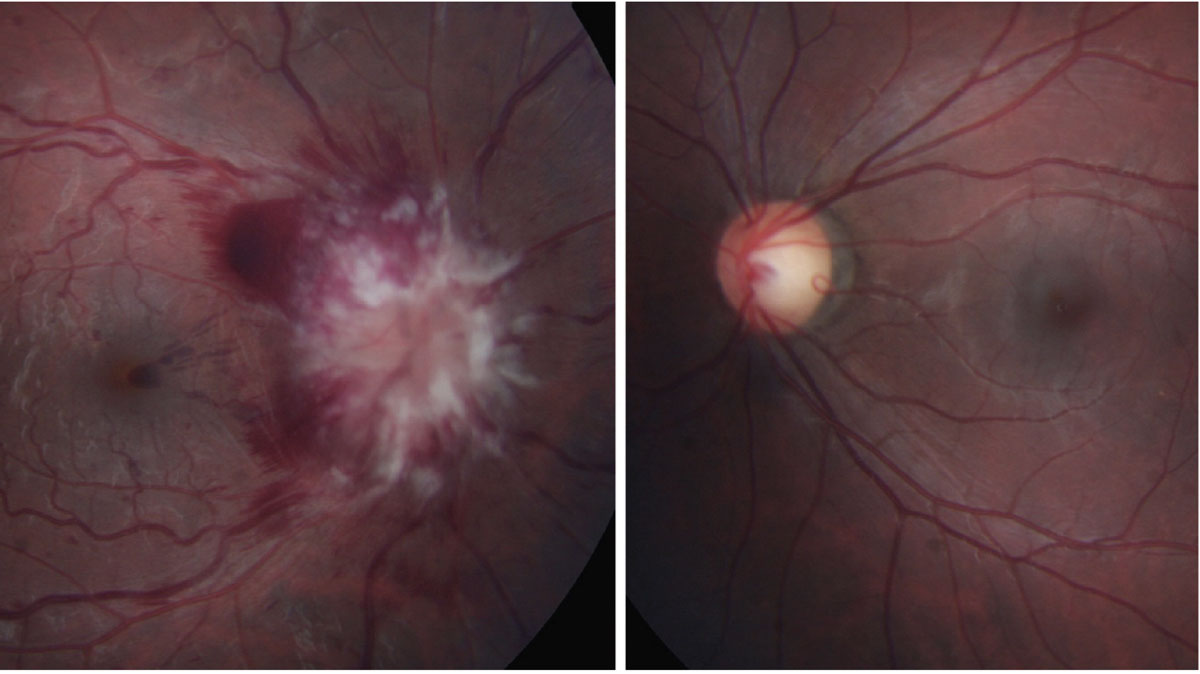 |
Fig. 8. Leukemic infiltrative optic neuropathy. Click table to enlarge. |
Hereditary
Leber’s hereditary optic neuropathy has a mitochondrial inheritance pattern and thus a predilection toward men in their 20s and 30s. They present with symptoms of painless, bilateral vision loss over the course of days or weeks. Visual field testing shows dense central scotomas and funduscopy may show peripapillary telangiectasia (Figure 9).19 Family history typically reveals generations of blindness and can be confirmed with genetic testing. Unfortunately, visual prognosis for these patients is poor (counting fingers vision or worse) and they should be set up with low vision rehabilitation services. A cardiology referral is also indicated due to the associated risk for cardiac conduction abnormalities.19
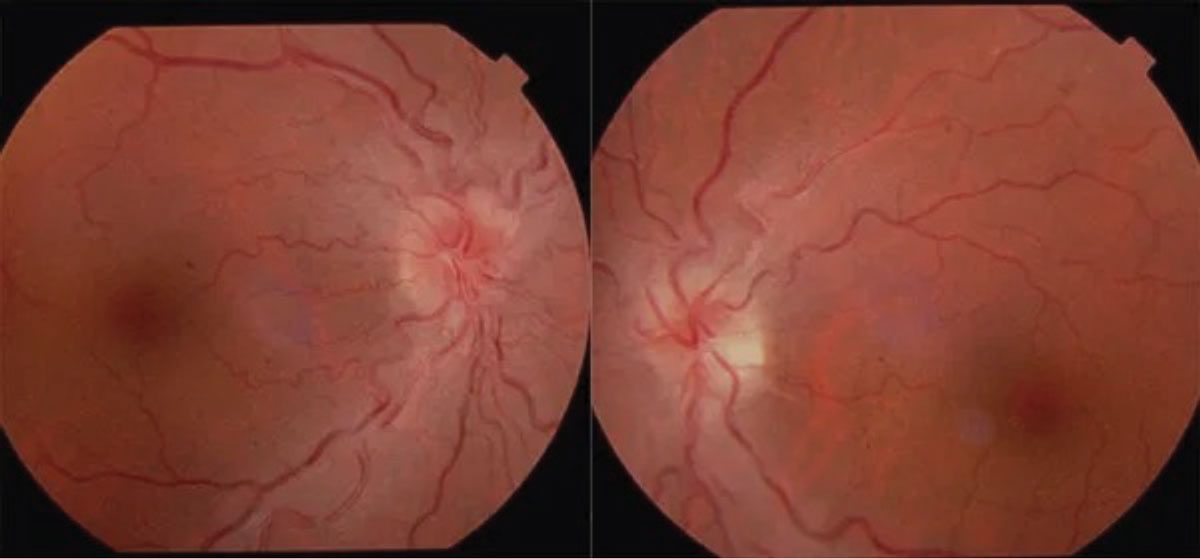 |
Fig. 9. Leber’s hereditary optic neuropathy. Click table to enlarge. |
Takeaways
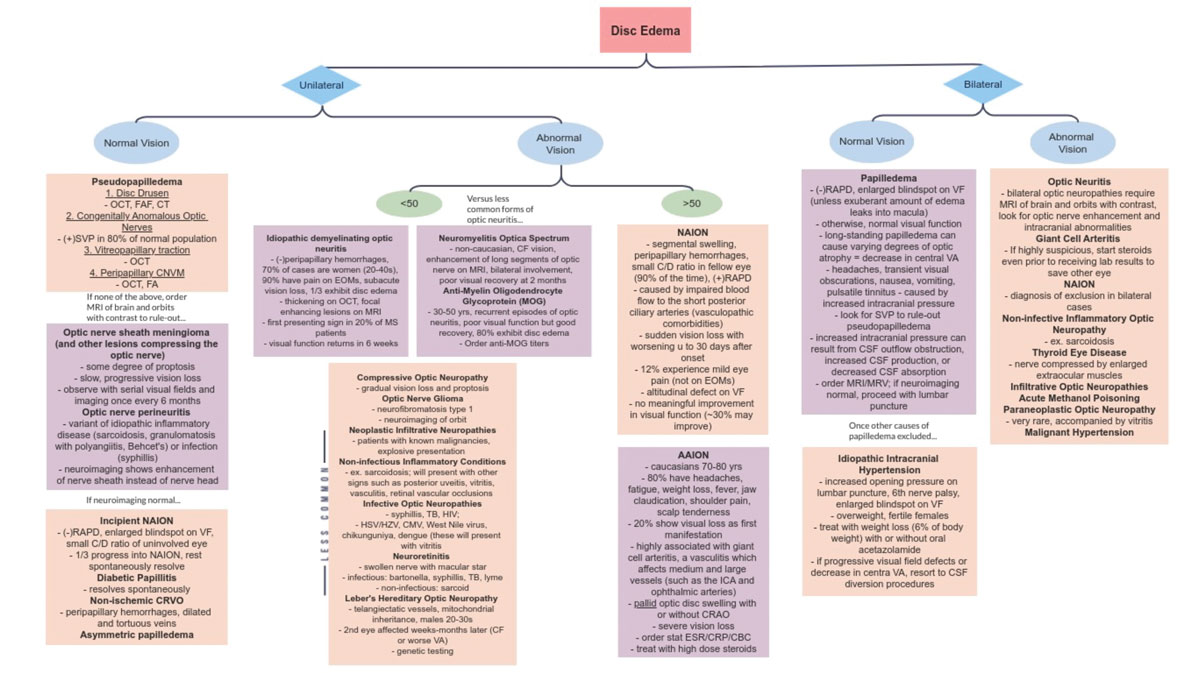
Hopefully with this information in your cache, the next in-office presentation of disc edema will be less nerve-wracking. Knowing the differentials, their risk factors and associated signs and symptoms can help narrow down your diagnosis (Figure 10). While reaching the correct diagnosis is important, management of optic atrophy also matters. As a result, getting as much baseline testing as possible in the initial visits of non-emergent cases would be beneficial for long-term follow-up.
 |
|
Fig. 10. Flow chart of disc edema differentials. Click image to enlarge. |
Dr. Komma is an optometrist at Guilford Eye Center in Greensboro, NC. She graduated from the Pennsylvania College of Optometry and completed her ocular disease residency at the Salisbury VA Medical Center. She served as an externship coordinator for the Kernersville VA Healthcare Center and is a Fellow of the American Academy of Optometry. She is the current president of the NCAAO. She has no financial interests to disclose.
1. Dhoot R, Margolin E. Papilledema. PubMed. In: StatPearls. August 8, 2023. Accessed November 16, 2023. https://www.ncbi.nlm.nih.gov/books/NBK538295/. 2. Bhakhri R, Luce C. Optic disc drusen and associated complications: a teaching case report. The Journal of Optometric Education. Accessed November 16, 2023. https://journal.opted.org/article/optic-disc-drusen-and-associated-complications-a-teaching-case-report/. 3. Silverman AL, Tatham AJ, Medeiros FA, Weinreb RN. Assessment of optic nerve head drusen using enhanced depth imaging and swept source OCT. J Neurophthalmol. 2014;34(2):198-205. 4. Moret F, Poloschek CM, Lagrèze WA, Bach M. Visualization of fundus vessel pulsation using principal component analysis. Invest Opthalmol Vis Sci. 2011;52(8):5457. 5. Thurtell MJ, Wall M. Idiopathic intracranial hypertension (pseudotumor cerebri): recognition, treatment and ongoing management. Curr Treat Options Neurol. 2012;15(1):1-12. 6. Park MG, Roh J, Ahn SH, et al. Papilledema and venous stasis in patients with cerebral venous and sinus thrombosis. BMC Neurology. 2023;23(175). 7. Thon OR, Gittinger JW. Medication-related pseudotumor cerebri syndrome. Semin Ophthalmol. 2016;32(1):134-43. 8. Purvin VA, Kawasaki A, Yee RD. Papilledema and obstructive sleep apnea syndrome. Arch Ophthalmol. 2000;118(12):1626-30. 9. Weiner G. Case Studies of Optic Disc Edema. American Academy of Ophthalmology. October 1, 2015. Accessed January 13, 2024. www.aao.org/eyenet/article/case-studies-of-optic-disc-edema. 10. Jung JJ, Baek H, Analysis of the causes of optic disc swelling. Kor J Ophthalmol. 2011;25(1):33-6. 11. Margolin E. The swollen optic nerve: an approach to diagnosis and management. Prac Neurol. 2019;19(4):302-9. 12. Bernstein SL, Johnson MA, Miller NR. Nonarteritic antrior ischemic optic neuropathy (NAION) and its experimental models. Prog Retin Eye Res. 2011;30(3):167-87. 13. Raizada K, Margolin E. Non-arteritic anterior ischemic optic neuropathy. PubMed. October 31, 2022. Accessed January 13, 2024. www.ncbi.nlm.nih.gov/books/NBK559045/. 14. Giuliari GP, Sadaka A, Chang PY, Cortez RT. Diabetic papillopathy: current and new treatment options. Curr Diabetes Rev. 2011;7(3):171-5. 15. Gupta S, Sethi P, Duvesh R, et al. Optic perineuritis. BMJ Open Ophthalmol. 2021;6(1):e000745. 16. Purvin V, Sundaram S, Kawasaki A. Neuroretinitis: review of the literature and new observations. J Neuroophthalmol. 2011;3(1):58-68. 17. Rodriguez-Beato FY, De Jesus O. Compressive optic neuropathy. PubMed. August 23, 2023. Accessed January 13, 2024. www.ncbi.nlm.nih.gov/books/NBK560583/. 18. Compressive and Infiltrative Optic Neuropathies | Walsh and Hoyt Textbook. November 16, 2023. Accessed January 13, 2024. https://collections.lib.utah.edu/details?id=190040. 19. Shemesh A, Sood G, Margolin E. Leber Hereditary Optic. PubMed. September 24, 2022. Accessed January 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482499/. |

