A very somber, elderly woman brightened my day when she complained about the sudden appearance of wrinkles on her face following cataract surgery. She told me that, as she continued to look into her bathroom mirror, she was further horrified by a previously undetected cobweb located above the shower.
While humorous, it is gratifying to hear the many ways that patients express excitement about improved vision following cataract surgery. Under normal circumstances, cataract extraction (CE) is a low-risk procedure. Infrequently, some patients may experience significant postoperative complications, such as uveitis or endophthalmitis. However, one potential CE complication that rarely garners coverage in the ophthalmic literature is nonarteritic anterior ischemic optic neuropathy (NAION).
The incidence of NAION following CE is estimated at one case per 2,000 procedures.1 However, multiple studies have suggested that just 2.3 to 10.2 cases per 100,000 incidences of spontaneous NAION actually are reported in the general population over age 50.2,3
This article reviews two cases of NAION following CE. The first patient experienced NAION following CE in the first eye, and then developed NAION in the contralateral eye following a subsequent CE procedure years later. The patient was very fortunate that her second eye was not as visually compromised as the first.
The second patient underwent CE in one eye and then developed NAION years later. But, because of a longstanding fear of NAION in the second eye, she delayed the second cataract procedure for more than seven years.
Case I
History
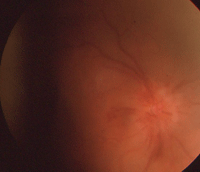
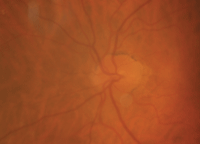
The right eye of our 87-year-old patient described in Case I. This image was taken one month following cataract extraction. Note the presence of hyperemia, hemorrhages and edema.
An 87-year-old female presented for a cataract surgery consultation. The patient said that she wanted to undergo CE in her left eye in order to retain an unrestricted Pennsylvania driver’s license. Her ocular history was significant for cataract removal with a posterior chamber intraocular lens (IOL) implantation in her right eye five years earlier. Two weeks after implantation, she developed NAION in her right eye.
Her visual acuity measured 20/50 OD at the three-day postoperative visit; however, four weeks later, her acuity was reduced to finger counting. She was placed on a short course of oral prednisone, which was discontinued after one week following the onset of NAION.
Diagnostic Data
At the CE consultation for her left eye, our patient’s uncorrected visual acuity measured 20/300 OD and 20/70 OS with pinhole testing. Her spectacle-corrected visual acuity measured 20/60 OU. She correctly identified 0/7 plates OD and 7/7 plates OS on color vision testing.
Her intraocular pressure measured 20mm Hg OD and 18mm Hg OS at 10:00 a.m. Pupillary testing uncovered a grade +2 afferent defect OD, but was unremarkable OS. Confrontation fields demonstrated a central/inferior scotoma OD but were full OS.
Slit lamp examination of the right eye revealed a clear anterior chamber and cornea, and a well-centered IOL implant. The left eye exhibited a grade 3 nuclear sclerotic cataract.
Fundus examination of the right eye showed a pale optic nerve head, with a cup-to-disc ratio of 0.30 x 0.30. We noted mild pigmentary changes and a 3DD choroidal nevus located temporal to the macula. All other findings were normal. Fundus examination of the left eye revealed a pink and healthy optic nerve, with a cup-to-disc ratio of 0.35 x 0.35. All other findings were normal. Refraction measured -1.00D -2.00D X 100 OD and -1.00D -1.25D X 085 OS.
Current systemic medications included 81mg aspirin per day, which she began using when diagnosed with NAION in her right eye after the first CE procedure. Her systemic history was unremarkable.
Management Plan
In consultation with both the patient and her son, we scheduled her for CE OS. In accordance with Pennsylvania requirements, we advised the patient that until she underwent CE, she was not permitted to drive at night.
Follow-up and Discussion
• One week. She underwent successful CE OS, and reported good vision and no pain at her one-week follow-up. She reported excellent compliance with her topical antibiotic, NSAID and steroid; however, she missed four days of aspirin use. We documented small optic nerve head splinter hemorrhages with no disc edema in the left eye.

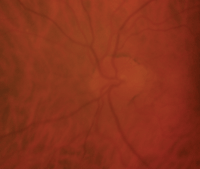
Our patient’s right eye at the two-month post-op follow-up. Although some hemorrhages were still present, the hyperemia and edema decreased.
• One month. Her uncorrected visual acuity measured 20/300 OD and 20/70 OS with pinhole testing. (Pinhole yielded no improvement OD; however, her bilateral acuity improved to 20/40 without spectacle correction.) She correctly identified 0/7 plates OD and 7/7 plates OS on color vision testing.
Her intraocular pressure measured 18mg Hg OU. Pupillary testing uncovered a grade +2 afferent defect OD, but was unremarkable OS. Confrontation fields demonstrated a central scotoma located at 20° OD, but were full OS.
Slit lamp examination of the right eye revealed a clear anterior chamber and cornea, and a well-centered IOL implant with no posterior chamber opacification (PCO). The left eye had trace cells in the anterior chamber and a clear cornea, with a well-centered IOL and no PCO.
Fundus examination of the right eye showed a pale optic nerve head OD, with a cup-to-disc ratio of 0.30 x 0.30. We noted mild dry pigmentary changes in the macula and a 3DD choroidal nevus located temporal to the macula. All other findings were normal.
Fundus examination of the left eye revealed splinter hemorrhages and segmental edema, with a cup-to-disc ratio of 0.35 x 0.35. All other findings were normal. Refraction measured -1.00D -2.00D X 100 OD and -0.75D -1.75D X 085 OS.
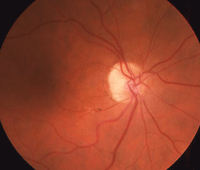
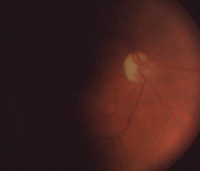
Our patient’s right eye at one-year (left) and three-year follow-up.
• Five months. The patient reported stable vision in her left eye, with no other visual or ocular complaints. Her final visual acuity measured 20/300 OD, 20/25 OS and 20/25 OU with spectacle correction.
Neuro-ophthalmic consultation was ordered after the splinter hemorrhages were detected, and confirmed the development of NAION in her left eye without giant cell arteritis.
While she achieved 20/25 OS on a high-contrast chart, she reported feeling “visually restricted.” Consequently, the patient stopped driving because she felt unsafe on the road.
The patient moved out of the area shortly after the five-month follow-up, and has not returned to our office since.
Case II


The right and left eyes of our 79-year-old patient described in Case II (OD top,
OS bottom). The images were taken before she developed NAION in her right eye.
History
Two weeks before a scheduled eye examination, a 79-year-old female presented with decreased vision in her right eye upon awakening. She denied concurrent headache, fatigue, temporal scalp tenderness and pain with jaw claudication.
Her ocular history was significant for uncomplicated CE in her right eye four years earlier. Additionally, she was being followed as an ocular hypertensive; however, her IOP never exceeded 24mm Hg. Her systemic history was remarkable for hypertension, arrhythmia and arthritis.
Current medications included 0.25mg digoxin, 40mg Nexium (esomeprazole magnesium, AstraZeneca), 20mg Lexapro (escitalopram oxalate, Forest Laboratories, Inc.), 50mg Cozaar (losartan, Merck), 81mg aspirin and an unconfirmed dosage of tramadol.
Diagnostic Data
Visual acuity measured light perception OD and 20/40 OS with pinhole testing. (Pinhole yielded no improvement OD.) Her spectacle-corrected visual acuity measured 20/40 OU. She correctly identified 0/7 plates OD and 7/7 plates OS on color vision testing.
Her intraocular pressure measured 18mm Hg OU. Pupillary testing uncovered a grade +2 afferent defect OD and a grade 2 nuclear sclerotic cataract OS. Confrontation fields demonstrated inferior loss OD, but were full OS.
Slit lamp examination of the right eye showed a clear anterior chamber and cornea, and a well-centered IOL with no evidence of PCO. The left eye showed a grade 2 nuclear sclerotic cataract.
Fundus examination of the right eye revealed disc edema with hemorrhages and cotton-wool spots, as well as mild drusen formation and macular pigment changes. The cup-to-disc ratio measured 0.30 x 0.30 OD. Fundus examination of the left eye revealed mild drusen formation and macular pigment changes. The cup-to-disc ratio measured 0.25 x 0.25 OS. Refraction measured 2.25D -2.75 X 100 OD and 2.50D -2.75D X 090 OS.
Management Plan
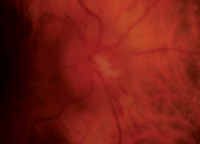
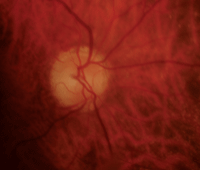
Our patient’s right and left eyes (OD top, OS bottom) two weeks after she experienced NAION OD. Note the splinter hemorrhage, hyperemia and edema in her right eye.
We ordered immediate blood testing, including sedimentation (SED) rate and C-reactive protein. Although the SED rate was normal—considering the patient’s age, optic disc appearance and precipitous vision loss—the neuro-ophthalmologist performed a temporal artery biopsy. It was negative.
We have followed the patient for seven years since she developed NAION in her right eye. Fortunately, no changes have occurred to the left optic nerve. Because of the complication in her right eye, and the consequently increased potential for NAION in the contralateral eye, the patient decided not to undergo CE in her left eye.
Signs and Symptoms of NAION
There are two forms of anterior ischemic optic neuropathy, arteritic and nonarteritic. Of these, NAION is less visually devastating and, as we shall discuss, can be associated with CE.
As clinicians, we are familiar with the NAION patient who reports sudden, painless, monocular vision or visual field loss. Often, these losses are noticed when waking up. Pain has been reported upon eye movement, but is extremely rare.4 Acuity may vary from no change to extreme visual compromise. Visual field loss also can be variable, but typically manifests as an altitudinal deficit or arcuate scotoma.
A relative afferent pupillary defect is always present in patients who present with NAION.5 Clinical signs include the familiar swollen disc with hyperemia as well as peripapillary, flame-shaped hemorrhages.6 These findings sometimes can involve just a small segment of the disc, as seen in the left eye of the patient described in Case I.
Both eyes typically show what is termed a “disc at risk,” with a small central cup. In most instances, the patients have an associated systemic history of diabetes mellitus, hypertension, hypercholesterolemia or a combination of these conditions.7
In addition to CE, other conditions that may act as triggers for NAION are nocturnal hypotension, obstructive sleep apnea, anemia, hyperhomocysteinemia and some coagulopathies.8,9
Phosphodiesterase type 5 inhibitors (PDE5 inhibitors), such as Viagra (sildenafil, Pfizer), also may precipitate NAION.10 Interestingly, however, a recent literature review in the Journal of Sex Medicine indicated that no conclusive evidence supports a direct cause/effect relationship between PDE5 inhibitors and vision-threatening ocular events.11
CE and NAION
The mechanism for the development of an optic neuropathy following CE may be related to IOP elevation. Sanjay S. Hayreh, MD, PhD, and associates suggest that elevated IOP secondary to CE causes a disruption in ocular perfusion pressure of the posterior ciliary arteries—either directly or via the peripapillary choroid.12,13 This, in turn, results in ischemia and a cascade of edema that leads to optic nerve head swelling.12,14
Interestingly, other clinicians have documented NAION following CE in patients who exhibited good IOP control.15 Transient IOP increase during the operative or perioperative period may be responsible for causing the ischemia.
As seen in Case I, NAION was closely associated with CE. There have been numerous reports of a causative association, including surgical technique.1,16 For optometrists, the important consideration is that if NAION has occurred in one eye—either following CE or spontaneously—the risk of the second eye developing NAION following CE is high.1
In a review of nearly 6,000 CE cases from the Bascom Palmer Eye Institute in Miami, three patients developed NAION within one year of surgery. Two of the three individuals had a history of NAION in the contralateral eye.1
Three months after our patient developed NAION in her right eye (OD left, OS right). Note that her right optic nerve was very pale, yet retained nearly the same cup-to-disc ratio.
More alarmingly, another study indicated that 53% of CE patients who had a previous NAION developed a subsequent NAION following CE in the contralateral eye.6 This finding suggests that CE patients with a history of NAION are nearly four times more likely to experience optic neuropathy than those with no previous history of NAION.
Both clinicians and researchers generally agree that NAION following CE usually occurs within hours or days of surgery. Whether CE can cause delayed NAION weeks to months later is more controversial.
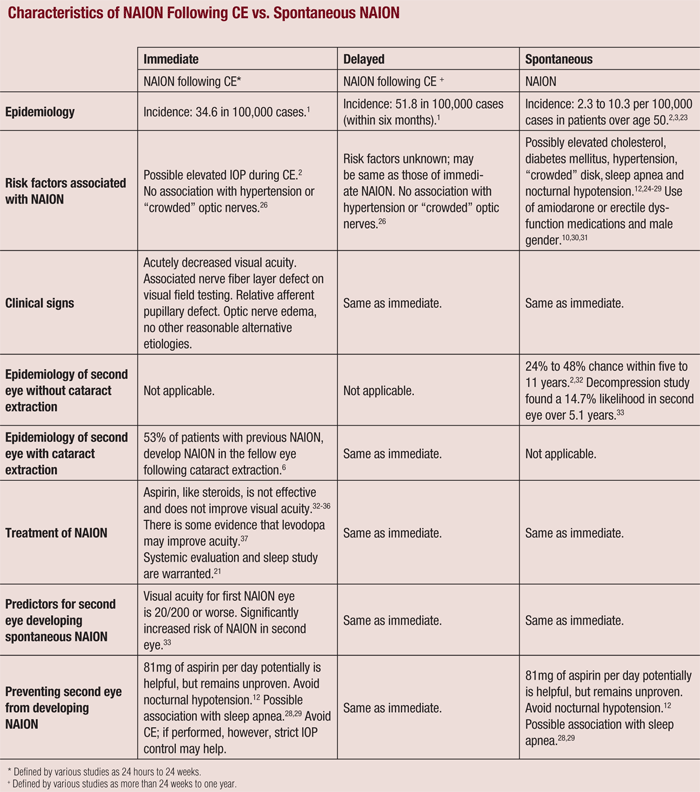
While the incidence of NAION following CE is relatively low, it is more likely to occur than spontaneous NAION in patients who have not undergone CE.1,12 Also, if delayed NAION was not associated with CE, but merely spontaneous instead, an equal distribution of postoperative cases would be expected during the year following surgery (see “Characteristics of NAION Following CE vs. Spontaneous NAION.”).17
Additionally, the clinical findings for CE-induced and spontaneous NAION also are markedly different. For example, patients with postoperative NAION typically have significantly lower rates of hypertension and higher cup-to-disc ratios.14
Despite this evidence, one article in the Journal of Cataract and Refractive Surgery indicated that there is insufficient data to confirm a relationship between CE-induced and delayed NAION.18
Treatments for NAION
With no treatment, visual acuity can improve for up to one year in approximately 50% of cases.19 In the Ischemic Optic Neuropathy Decompression Trial, surgical intervention to save the optic nerve was found to be ineffective, and even potentially harmful.19
Other interventional strategies have been attempted to further increase this recovery period, including daily aspirin use, attempts to improve optic nerve perfusion pressure, vasodilators, systemic steroids and intravitreal steroids. Unfortunately, none of these treatments have been proven to be effective in clinical trials.20
Increased Risk of Stroke and Heart Attack
Any patient who develops NAION is at an increased risk for cerebrovascular and cardiovascular events.21 You should promptly inform the individual’s primary care provider of the diagnosis, and educate the patient about his or her increased risk for the systemic conditions.
Further, if the patient is hypertensive, recommend that patients take their medications in the morning or during the day to avoid nocturnal hypotension. You may even wish to help enroll the patient in a study to evaluate for sleep apnea.
Patient Counseling
So, what guidance should you provide for a patient with a history of NAION and cataract? There are several fundamental considerations: What was the extent of vision loss secondary to NAION? Which eye is most visually dominant? What are the patient’s visual demands? What systemic and ocular comorbidities exist? How much does the existing cataract affect the patient’s vision and functional abilities?
In my office, we evaluate these factors concurrently during the patient education process. If a patient previously developed NAION following CE, inform the patient that he or she has a 53% likelihood of developing NAION in the fellow eye following CE.6 Further, explain that there is a 15% chance of developing NAION within five years, even if the second CE is not peformed.22 Finally, caution every patient that there are no effective treatment options if NAION develops in the contralateral eye.
Perhaps, within the next decade, an effective treatment for NAION will be developed. Until then, advise patients with a history of NAION to delay subsequent cataract procedures for as long as possible. Ultimately, of course, the decision to undergo contralateral CE is up to the patient. It is our responsibility to help the patient make this decision in light of the most current research.
Dr. Ruskiewicz is in private practice in Pottstown, Pa., and serves as an associate clinical professor at the Pennsylvania College of Optometry at Salus University in Elkins Park, Pa.
1. McCulley TJ, Lam BL, Feuer WJ. Incidence of nonarteritic anterior ischemic optic neuropathy associated with cataract extraction. Ophthalmology. 2001 Jul;108(7):1275-8.
2. Johnson LN, Arnold AC. Incidence of nonarteritic and arteritic anterior ischaemic optic neuropathy: population-based study in the state of Missouri and Los-Angeles County, California. J Neuroophthalmol. 1994 Mar;14(1):38-44.
3. Hattenhauer MG, Leavitt JA, Hodge DO, et al. Incidence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1997 Jan;123(1):103-7.
4. Swartz NG, Beck RW, Savino PJ, et al. Pain in anterior ischemic optic neuropathy. J Neuroophthalmol. 1995 Mar;15(1):9-10.
5. Chung SM. Nonarteritic Anterior Ischemic Optic Neuropathy. In: Levin LA, Arnold AC (eds.). Neuro-Ophthalmology: the Practical Guide. New York: Thieme; 2005:194-7.
6. Lam BL, Jabaly-Habib H, Al-Sheikh N, et al. Risk of nonarteritic anterior ischaemic optic neuropathy (NAION) after cataract extraction in the fellow eye of patients with prior unilateral non-arteritic anterior ischaemic optic neuropathy (NAION). Br J Ophthalmol. 2007 May;91(5):585-7.
7. Arnold AC. Pathogenesis of anterior ischemic optic neuropathy. J Neuroophthalmol. 2003 Jun;23(2):157-63.
8. Stein JD, Kim DS, Mundy KM, et al. The association between glaucomatous and other causes of optic neuropathy and sleep apenea. Am J Ophthalmol. 2011 Dec;152(6):989-98.e3
9. Worrall BB, Moazami G, Odel JG, Behrens MM. Anterior ischemic optic neuropathy and activated protein C resistance. A case report and review of the literature. J Neuroophthalmol. 1997 Sep;17(3):162-5.
10. Lee AG, Newman NJ. Erectile dysfunction drugs and non-arteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2005 Apr;140(4):707-8.
11. Azzouni F, Abu samra K. Are Phosphodiesterase type 5 inhibitors associated with vision-theatening adverse events? A critical analysis and review of the literature. J Sex Med. 2011 Oct;8(10):2894-903.
12. Hayreh SS. Anterior ischemic optic neuropathy IV: Occurrence after cataract extraction. Arch Ophthalmol. 1980 Aug;98(8):1410-6.
13. Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol. 1994 May 15;117(5):603-24.
14. McCulley TJ, Lam BL, Feuer WJ. A comparison of risk factors for postoperative and spontaneous nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2005 Mar;25(1):22-4.
15. Serrano LA, Behrens MM, Carroll FD. Correspondence-Postcataract extraction ischemic optic neuropathy. Arch Ophthalmol. 1982 Jul;100(7):1177-8.
16. Gass JD, Norton EW. Cystoid macular edema and papilledema following cataract extraction. Arch Ophthalmol. 1966 Nov;76(5):646-61.
17. McCulley TJ, Lam BL, Feuer WJ. Nonarteritic anterior ischemic optic neuropathy and surgery of the anterior
segment: Temporal relationship analysis. Am J Ophthalmol. 2003 Dec;136(6):1171-2.
18. Nguyen LT, Taravella MJ, Pelak VS. Determining whether delayed nonarteritic ischemic optic neruopathy associated with cataract extraction is a true entity. J Cataract Refract Surg. 2006 Dec;32(12):2105-9.
19. Ischemic Optic Neuropathy Decompression Trial Research Group. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA. 1995 Feb 22;273(8):625-32.
20. Atkins EA, Bruce BB, Newman NJ, Biousse V. Treatment of nonarteritic anterior ischemic optic neuropathy. Surv Ophthalmol. 2010 Jan-Feb;55(1):47-63.
21. Miller NR. Current concepts in the diagnosis, pathogenesis, and management of nonarteritic ischemic optic neuropathy. J Neuroophthalmol. 2011 Jun;31(2):e1-3.
22. Ellenberger C, Keltner JL, Burde RM. Acute optic neuropathy in older patients. Arch Neurol. 1973 Mar;28(3):182-5.
23. Repka MX, Savino PJ, Schatz NJ, Sergott RC. Clinical profile and long term implications of anterior ischaemic optic neuropathy. Am J Ophthalmol. 1983 Oct;96(4):478-83.
24. Moro F, Doro D, Mantovani E. Anterior ischaemic optic neuropathy and aging. Metab Pediatr Syst Ophthalmol. 1989;12(1-3):46-57.
25. Hayreh SS, Joos KM, Podhajsky P, Long CR. Systemic diseases associated with nonarteritic anterior ischaemic optic neuropathy. Am J Ophthalmol. 1994 Dec 15;118(6):766-80.
26. Jacobson DM, Vierkant RA, Belongia EA. Nonarteritic anterior ischaemic optic neuropathy: a case-control study of potential risk factors. Arch Ophthalmol. 1997 Nov;115(11):1403-7.
27. Salomon O, Huna-Baron R, Kurtz S, et al. Analysis of vascular and prothrombotic risk factors in patients with nonarteritic anterior ischaemic optic neuropathy (NAION). Ophthalmology. 1999 Apr;106(4):739-42.
28. Li J, McGwin G Jr, Vaphiades MS, Owsley C. Non-arteritic anterior ischaemic optic neuropathy and presumed sleep apnoea syndrome screened by the sleep apnea scale of the sleep disorders questionnaire (SA-SDQ). Br J Ophthalmol. 2007 Nov;91(11):1524-7.
29. Mojon DS, Hedges TR 3rd, Ehrenberg B, et al. Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol. 2002 May;120(5):601-5.
30. Murphy MA, Murphy JF. Amiodarone and optic neuropathy: the heart of the matter. J Neuroophthalmol. 2005 Sep;25(3):232-6.
31. Beri M, Klugman MR, Kohler JA, Hayreh SS. Anterior ischemic optic neuropathy, VII. Incidence of bilaterality and various influencing factors. Ophthalmology. 1987 Aug;94(8):1020-8.
32. Salomon O, Huna-Baron R, Steinberg DM, et al. Role of aspirin in reducing the frequency of second eye involvement in patients with non-arteritic anterior ischaemic optic neuropathy. Eye (Lond). 1999 Jun;13 ( Pt 3a):357-9.
33. Ischemic optic neuropathy decompression trial research group. Ischemic optic neuropathy decompression trial: twenty-four month update. Arch Ophthalmol. 2000 Jun;118(6):793-8.
34. Botelho P, Johnson LN, Arnold A. The effect of aspirin on the visual outcome of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1996 Apr;121(4):450-1.
35. Atkins EA, Bruce BB, Newman NJ, Biousse V. Translation of clinical studies to clinical practice: survey on the treatment of nonarteritic anterior optic neuropathy. Am J Ophthalmol. 2009 Nov;148(5):809.
36. Kelman SE. Intravitreal triamcinolone or bevacizumab for nonarteritic anterior ischemic optic neuropathy: do they merit further study? J Neuroophthalmol. 2007 Sep;27(3):161-3.
37. Johnson LN, Guy ME, Krohel GB, Madsen RW. Levodopa may improve vision loss in recent-onset nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2000 Mar;107(3):521-6.

