There’s no professional conference or discussion forum these days that ignores myopia and the need to control its development, but what happens in practice? The most recent data indicates that less than 20% of eyecare practitioners provide any method of myopia control.1 Worldwide, single vision lenses are still the most commonly prescribed, despite mounting evidence in the literature to support other interventions.2 Is this a collective denial of the myopia epidemic Brien Holden alerted us to a decade ago, or is there simply a disconnect between what we want to achieve and what we’re capable of achieving? With limited financial resources and even less free time, is the thought of reengineering our practices to make them suited to myopia management simply a bridge too far?
In this article, we will look at the evidence and try to translate findings from research into clinical practice. We’ll also tackle some of the most common myths that hold back the adoption of various myopia interventions. Perhaps it will then be possible to move the needle and increase the number of patients who benefit from effective myopia control.
 |
A 2023 survey of eyecare practitioners globally found conventional single vision spectacle lenses to be the most prescribed option for progressive myopia in children (32.2% of respondents). However, this has been declining since 2019, the report states, and myopia control spectacles (15.2%), myopia control contact lenses (8.7%) and combination therapy (4.0%) are growing in popularity. Click image to enlarge. |
Where Does Myopia Come From?
This is a fundamental question. We need to understand the factors that can trigger myopia and those that can cause it to progress, even in adulthood. With this understanding, it becomes easier to select control methods and, most importantly, understand their limitations so that we can set realistic expectations about their effectiveness.
Any first-year optometry student will tell you that myopia can be defined as a refractive error in which rays of light entering the eye parallel to the optical axis are focused in front of the retina when ocular accommodation is relaxed.3 This usually results from the eye being too long from front to back, but can also be caused by an overly steep cornea, a lens with increased optical power, or both. This is the common understanding of most eyecare professionals around the world. However, this definition fails to account for dynamic processes during growth that, importantly, we have the ability to influence.
On closer inspection, myopia is more a matter of a loss of retinal homeostasis that disrupts the balance achieved during emmetropization, resulting in an eye that is too long for its dioptric power.4 The refractive error, therefore, does not define the nature of the damage but rather its consequence.
In fact, as with other organs, the eye’s growth is regulated by homeostatic control mechanisms. In this case, the eye relies on the quality of the visual signal hitting the retina as a principal input to guide its growth.5 In this way, the retina can react to whether the image is clear or blurred. The retina we’re talking about here is the central retina (the foveal retina) and the one within 10° to 20° of the fovea (macular retina).6 Specifically, this is limited to the macular area (1°= 0.3mm; 15° to 20°= 4.5mm to 6.0mm).7,8 Outside this more sensitive zone, the retinal signal becomes noise that cannot be well interpreted by the retinal tissue, especially due to the asymmetry of the ocular structure between the different quadrants.9
 |
Click image to enlarge. |
A blurred image that forms in front of the peripheral retina is referred to as myopic defocus, while a blurred image that forms behind the retina is referred to as hyperopic defocus. The former causes the eye to resist elongation, while the latter favors an increase in axial length and thus greater myopization.10
When we talk about defocus, we mean optical aberrations produced by spectacle lenses, or, in the case of ortho-K, by the shape of the cornea. Myopic defocus is due to a more convex power and is therefore associated with positive spherical aberrations and coma, whereas hyperopic defocus is associated with negative aberrations, as found in single-vision spectacle lenses. More specifically, when positive aberrations increase significantly (which is the goal in myopia management), they cause a decrease in contrast sensitivity to the point where the retina becomes confused. This is what slows down the development of myopia.11
Similarly, in emmetropia the presence of one type of defocus theoretically balances the presence of the other, and retinal homeostasis is reached.12 The retina, thus exposed to conflicting stimuli, remains neutral and aligned with its dioptric power. When one of the two signals becomes dominant, the retina resumes its resistance to elongation (e.g., in the presence of multifocal/bifocal contact lenses) or, conversely, favors axial length increase (wearing single-vision minus powered lenses).
All this happens through the release of biomodulators that affect the blood flow and the choroid, then the sclera. In the presence of myopic defocus, biomodulators thicken the choroid and lead to collagen fibers remodeling to make sclera stiffer.13 With hyperopic defocus, the choroid thins and blood flow is reduced, causing hypoxia. The scleral fibers remodel, with the tissue becoming softer and more easily deformable.14 It’s important to remember that the central retina is not just passive. Foveal blur is interpreted as form deprivation and leads to axial elongation.15 In all of these mechanisms, we must remember that each retina is unique, with its own threshold of stimulation.16 It also operates under a dose-response phenomenon.17
Goal of Myopia Management
Once this first step of understanding the mechanisms underlying myopia has been achieved, it’s time to set realistic goals for intervention methods. Keep in mind that the physical growth of a child, and therefore the growth of the eye, generates an axial elongation, especially during growth spurt.19 We can’t prevent this from occurring. Consequently, the goal must be to mimic the eye growth of an emmetropic patient whose retina is in homeostasis. Studies have shown that, across all ethnic groups, a mean increase in axial length of 0.2mm between the ages of five and 10, 0.1mm between the ages of 10 and 16, and no change thereafter is the norm for emmetropes.20 These limits should become our reference criteria for judging the myopic evolution of patients.
Time to Take Action
The need to intervene is well-established. Now, it’s time to take action. Let’s explore what tools we have in hand.
Depending on where in the world you practice, access to myopia control products may vary greatly. It’s understandable that a practitioner with access to limited resources might feel deprived. On the other hand, even if the treatment prescribed is not optimal, at least that professional is making an effort to correct myopia by understanding the underlying issues. There is always a way to help a child.
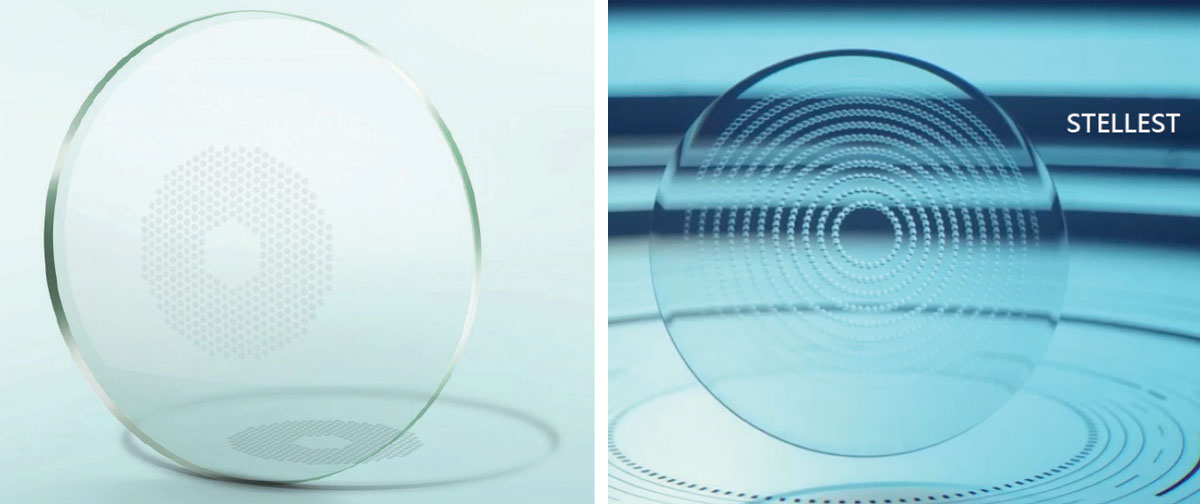 |
The Miyosmart lens from Hoya (left) contains a 9.4mm diameter central zone of distance vision surrounded by a honeycomb-like pattern of 396 defocusing lenslets of +3.50D power, equally distributed. Essilor’s Stellest (right) is designed with a similar 9mm central zone. However, the surrounding is made of highly aspherical lenses, varying in distribution and power vs. distance refractive error correction, along 11 segmented rings, to generate a volume of defocus following the retinal shape. Click image to enlarge. |
Basic Principles
Based on what we know about the mechanisms leading to myopia and its evolution, it makes sense to consider any device through the angle of their optical and physiological impacts. In order to select the best approaches, the following principles are important to consider:
1. Take the time to complete a thorough case history:
• rapid progression factors (age, sex, ethnicity, genetics, binocular vision)
• patient’s visual needs
• environment (screen time, working distance, outdoor exposure)
• willingness to wear contact lenses
• factors that may influence compliance (sleep time, sports and other activities)
• parents’ and patient’s preferences
• budget
2. Make sure to collect valid and accurate clinical data.
3. Select the strategy based on the risk of high myopia and long-term pathology. I strongly recommend the use of percentile growth charts according to ethnicity.25,26 These tables indicate, according to age and axial length, the expected progression as well as the risk of high myopia, and therefore of greater pathology. Thus, any age/axial length combination that exceeds the 50th percentile merits active control of the myopic condition. If this combination exceeds 75th percentile, more intensive measures should be considered.
For example: two kids, 10 years old, both -2.00D myopes, same school, same neighborhood, same video games, same everything. Child A shows axial length of 23.1mm and child B has a longer eye: 24.5mm. The first one reaches barely the 50th percentile associated with no high myopia risk and the former one is peaking at the 95th percentile with 16% chance of high (blinding) myopia. Strategy and treatment will not be the same to keep both safe. In the first case, anti-myopia glasses may be enough while a combined therapy with low-dose atropine will be needed for the kid at higher risk.
4. Select the optimal dose of defocus without impairing distance vision. The defocus dose is determined by the amount of positive optical aberrations produced and the area it covers in the macular area. The dose can be increased by using more convex power lenses and/or concentrating the myopic defocus in an area as close to the fovea as possible (10° to 20°). A higher dose is required for fast progressors (usually younger patients eight to 12 years old) and higher myopes.
5. Choose a strategy that meets the patient’s needs, but more importantly, one that is supported by evidence-based results from state-of-the-art, long-term randomized clinical trials. Be critical when reading articles or reports about the effectiveness of the product.
6. Ophthalmic lenses or pharmaceutical agents should be fitted and centered, or prescribed, according to the manufacturer’s recommendations.
7. Recommend a wear schedule for prescribed ophthalmic lenses.
8. Re-evaluate treatment periodically.
 |
Tools in Our Hands
These will vary depending on where you practice. As most of this publication’s readership is based in the US, you unfortunately do not yet have access to some interventions, most notably spectacle lenses designed for myopia control.
The remainder of this article will delve into the nuts and bolts of using various optical and pharmaceutical interventions.
 |
Spectacles
Anti-myopia spectacle lenses are undoubtedly the simplest way to equip a patient for myopia control. Several designs have been introduced to the market (four or five in Europe and Canada, and more than 20 to 30 in Asia), and their effectiveness is generally equivalent to that of other strategies, at least if the following elements are respected:27
• The frame chosen allows the eye to be well centered, in the middle, leaving sufficient room for defocus to hit the eye from all quadrants.
• The frame must be stable and not slip. If necessary, frames with nose pads and/or temples that can be easily adjusted to follow the curve of the ear are preferred.
• Spectacles should be worn for 12 to 14 hours, i.e., full time. This is undoubtedly the greatest challenge, and compliance can be sorely tested. Children wearing anti-myopia spectacles should not remove them when doing near work.
Table 1 shows differences between major products available in the market (outside US).
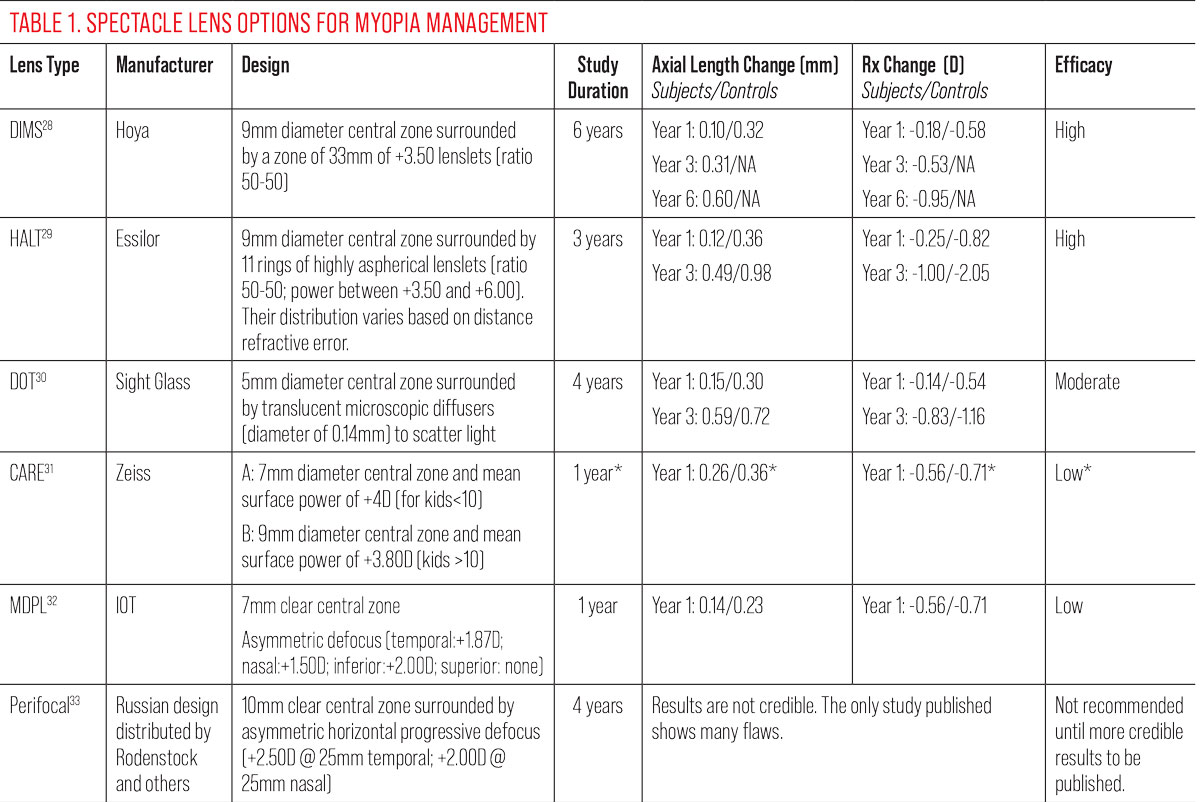 |
*Results are from a study conducted with a prototype lens very close to the final design, not the Zeiss CARE lens, for which data are unavailable. Results may vary with the lens designs as marketed. More data are needed from the manufacturer. Click image to enlarge. |
Contact Lenses
There are several contact lens options that can both correct and control myopic progression (Table 2). Remember that a sufficient dose of optical aberrations (defocus) must be generated to influence retinal response, while maintaining clear central vision. In general, these objectives can be achieved with multifocal lenses or by remolding the cornea with corneal lenses.34 Let’s concentrate on soft multifocal lenses.
Preferred soft lens designs are generally distance-centered multifocals, assuming that placing the convex profile (add power) in the periphery will make it easier to influence peripheral retinal response.
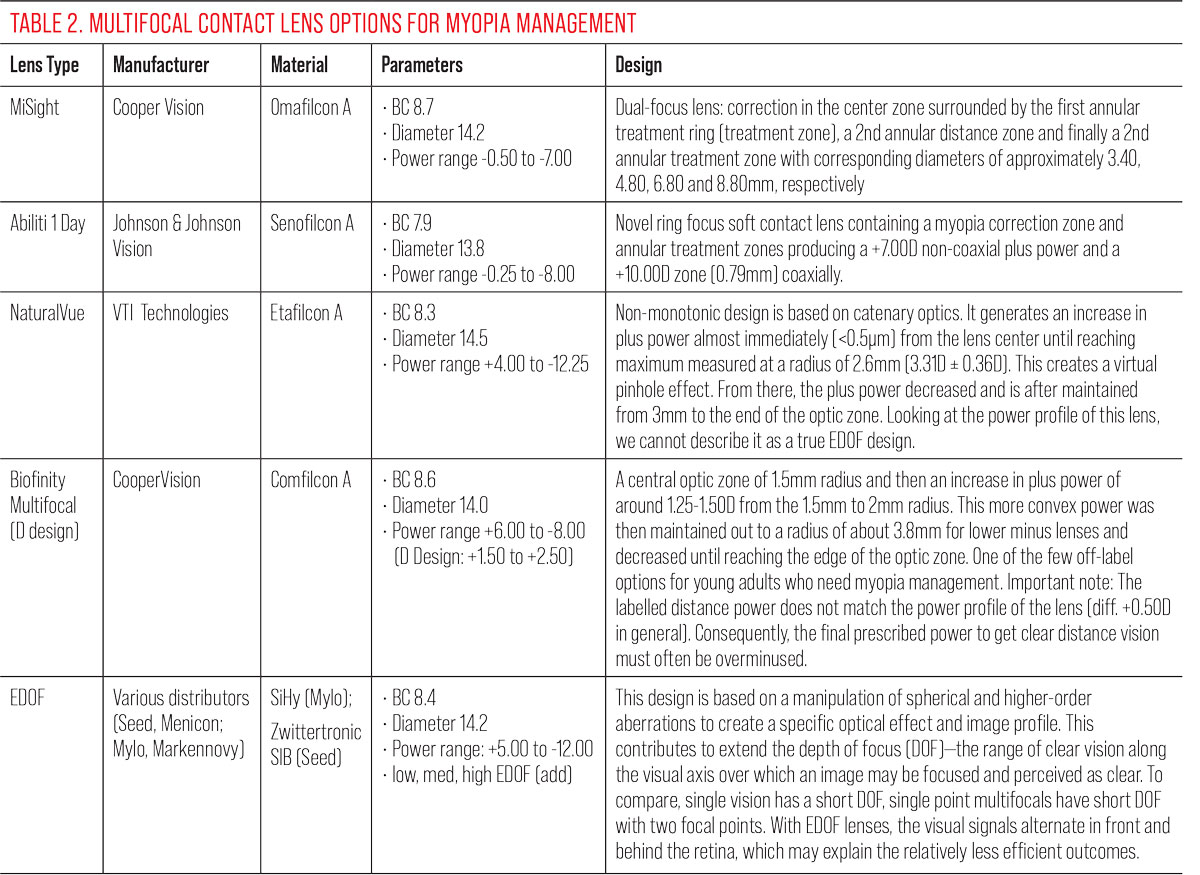 |
Table 3 describes the clinical population of several soft lenses studied in cohorts of young myopic patients.37-42 The populations among these studies are quite similar, with the exception of the Diaz-Gomez study of the Mylo EDOF lens, showing a slightly older and more myopic cohort at baseline. Table 4 summarizes the clinical performance of the various contact lenses.
Without a doubt, CooperVision’s MiSight 1 Day lens is the most studied and recognized anti-myopia contact lens in every region around the world. Its solid performance makes it the standard by which all others are measured. We only have short-term data on the other lenses, so comparison is difficult. At six months, Abiliti performs equally well to MiSight, but we cannot yet make a strong recommendation toward that product pending longer term results. At one year of wear, NaturalVue shows similarly good results. But again, longer term data will be needed to really compare all three products. Knowing that the best performance is always in the first year, it will be interesting to see if this favorable result continues over time.
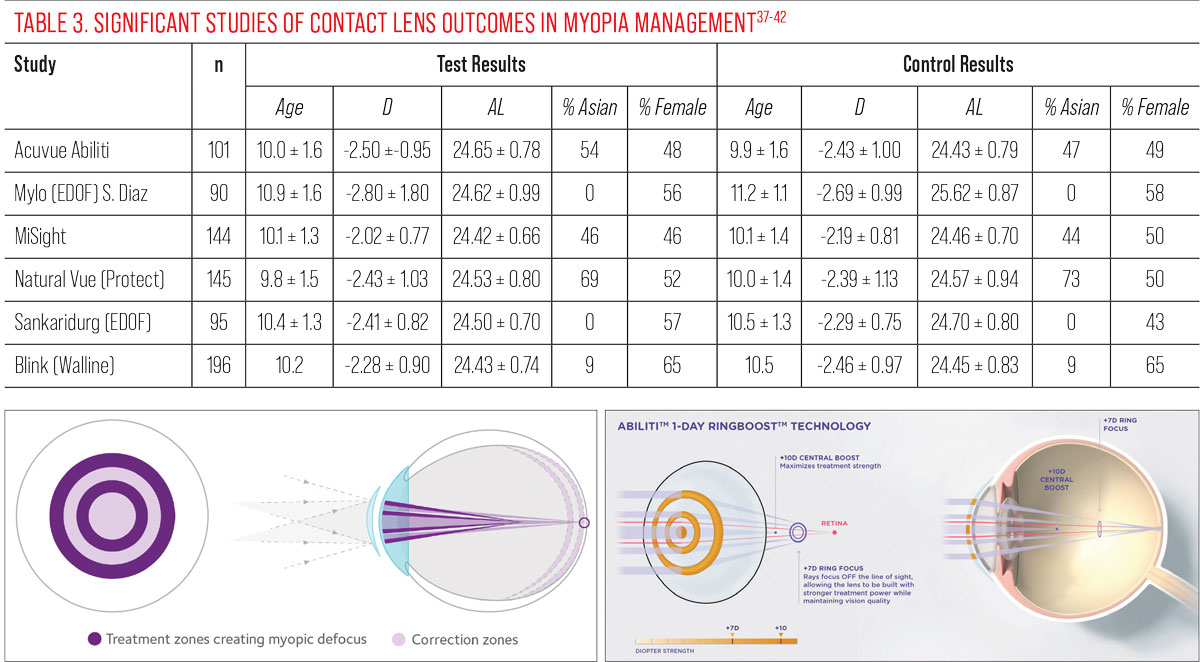 |
Both the Misight and the Acuvue Abiliti lenses seem to use the same alternating zones approach but, in fact, there are differences in the way they generate, and the level of, their respective myopic defocus (coaxial power and ring boost). |
Extended depth-of-focus (EDOF) designs, in some cases, may seem to be less efficient compared to other soft lens designs and best anti-myopia glasses.40 For example, after 36 months, EDOF lens wearers gained 0.55mm in axial length while MiSight wearers grew by 0.30mm during the same time. This is hard to explain, especially because the clinical population of EDOF is all Caucasian while the ethnic makeup is 50-50 Caucasian-Asian for studies of MiSight. If Asian myopic patients are progressing more, they must be increasing the rate rather than decreasing it. The EDOF study population is also older, so their rate of progression should be slower.
The numbers here are puzzling. Their control group shows a higher evolution vs. MiSight (0.97mm vs. 0.62mm). These suspect data echo the fact that the EDOF study reported an improvement in lens performance after the first year of wear, which is highly unusual in myopia studies. For all of these reasons, and given the significant difference from the early EDOF studies, the latest data must be interpreted with caution and the lens performance must be considered at least questionable.
Practitioners should also keep in mind the impact on a child’s life. Not only are contact lenses an effective means of correcting and controlling myopia, but fitting a child with contact lenses also boosts self-esteem and has been associated with psychological benefits.41 However, with contact lenses—as with anti-myopia spectacles—compliance is critical, both in terms of wearing days/hours to ensure a sufficient “dose” of control, and in terms of cleaning and disinfection, especially if ortho-K is recommended.
 |
Harrow Health offers three concentrations of atropine, although use of these (or other) formulations for myopia remains off-label at present in the United states. Vyluma continues to pursue FDA approval of its low-dose atropine product, code named NVK-002. Click image to enlarge. |
Atropine
Since the ATOM1 study, atropine has attracted considerable interest as a treatment for myopia. This study compared different concentrations of atropine administered monocularly over a two-year period in a cohort of young myopes. The results were surprising: The highest concentration (1%) was highly effective but associated with an equally high rebound effect, which was not expected. In contrast, the lowest concentration was surprisingly effective in controlling refraction to almost the same degree, while eliminating the rebound effect and significantly reducing the troublesome symptoms of light sensitivity and loss of accommodation.
This was enough for the authors to repeat a second study, called ATOM2, this time with lower concentrations only.43 They confirmed the relevance of considering atropine as a monotherapy in myopia control, at least for its effect on refraction. But nobody noticed at that time the very negligible effect of the 0.01% concentration on axial length, which continued to progress as much as the control group.
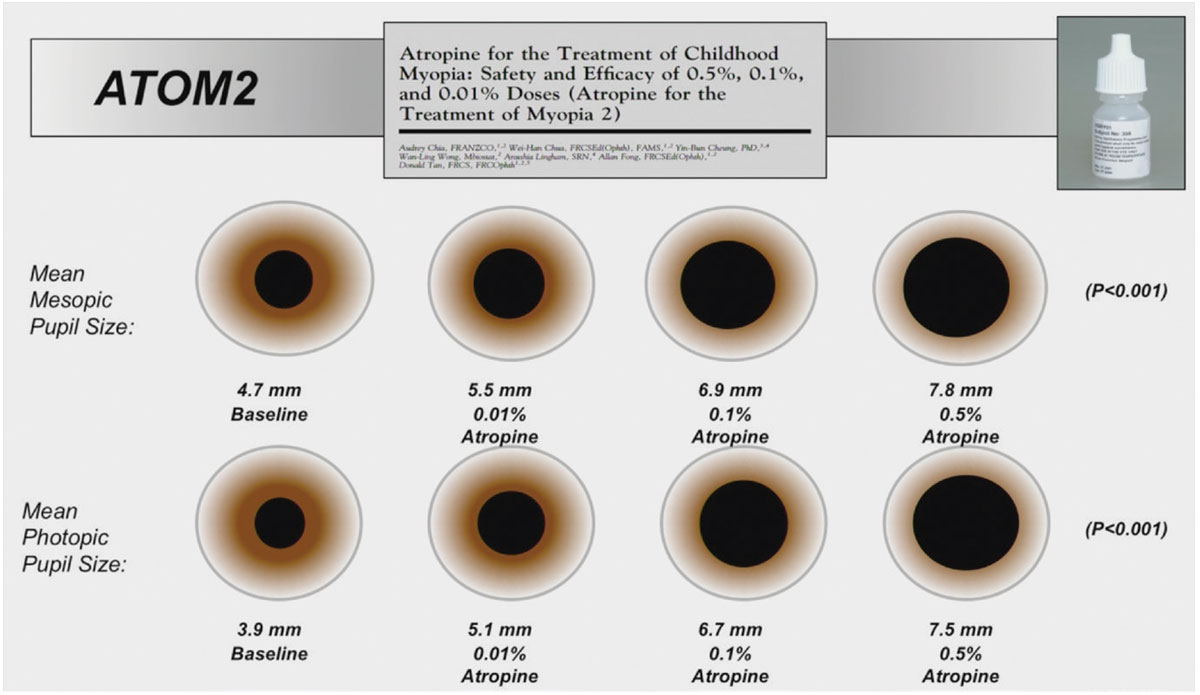 |
ATOM2 used lower concentrations of atropine to minimize side effects, including pupil dilation and associated photophobia, while maintaining effective control of myopic progression. This graph illustrates the expected variation in pupil size with different concentrations. It should be noted that there is a dose-response effect, both for pupil variation and for efficacy on axial length. Click image to enlarge. |
It should be noted that the authors of the ATOM studies revisited their patients 20 years later.44 They found that the treated patients had developed in the same way as the control group. Their treatment had no long-term effect. It has to be said that the ATOM participants were only treated for two years, while they were still young or teenagers and still evolving rapidly. That’s like treating a glaucoma patient for only two years and then looking at their eye health 20 years later, when they would have stopped treatment altogether. The damage to the optic nerve would be obvious.
Following ATOM, the LAMP study was initiated, again in Asia. This time, researchers compared reduced doses of atropine (0.05% vs. 0.02% and 0.01%). This study has just published its five-year results.45 The results are as follows:
• The study confirms a dose-response mechanism: the 0.05% concentration controls myopia better than the others.
• The 0.05% concentration may not be sufficient for some children, especially those progressing rapidly or under the age of 10. In these cases, a higher concentration is required.
• Brief discontinuation of the medication does not result in a significant loss of efficacy.
 |
In cases where the concentration is not sufficient, the Netherlands method should be used.46 This approach, which originated in Rotterdam, uses percentile growth charts as a starting point. Any patient above the 75th percentile is given a 0.5% dose of atropine. Of course, side effects must be compensated for by wearing progressive (add +1.75D) and photochromic glasses. This dose is maintained until the age of 16 years or earlier if stability is achieved (<0.05mm axial length progression per year for two consecutive years). At this concentration, the dose should then be tapered by halving the concentration every two to three months until discontinued. If axial length increases again, repeat the dose until it stabilizes. For patients below the 75th percentile, a dose of 0.05% is recommended. Again, treatment is continued until stabilization. Although not mandatory, it is recommended to taper the drug after discontinuation. This is consistent with a recent study that reported a potential rebound effect even with a reduced dose of 0.01%.
Other recent studies have examined the efficacy of the 0.01% concentration as monotherapy. These studies are almost unanimous in demonstrating the ineffectiveness of this dose, particularly in younger myopes who progress more rapidly.47-49 There are few exceptions like the CHAMP study, which compared 0.02% and 0.01% concentrations.50 The products used are standardized and not compounded preparations. This ensures product stability and the certainty of a constant dose. Surprisingly, the 0.01% concentration proved to be more effective than the 0.02% concentration in both aspects studied, i.e., refractive component and axial length.
 |
Takeaways
There is only one conclusion to this article. Myopia control is not optional, but rather a new standard of practice to prevent pathologies that can lead to visual handicap and long-term risks to eye health. The use of single-vision optical devices should be considered the exception rather than the rule. The methods used to control myopia have never been so abundant as they are now, and the pipeline is full of new products that will add to this armamentarium. Strategies and products must be tailored to the individual characteristics of the myopic patient (one child at a time) and be maintained until the condition has stabilized, which obviously requires regular follow-up over time.
Myopia is no longer a simple refractive error. It is, for the patient and for us, a rewarding journey.
Dr. Michaud is the former dean of the School of Optometry at the L’École d’optométrie de Université de Montréal. He is a diplomate of the American Academy of Optometry and a fellow of the British Contact Lens Association, the Scleral Lens Education Society, the European Academy of Optometry and the International Academy of Orthokeratology and Myopia Management. He was awarded the Donald R. Korb for excellence in research by the AOA and named the GPLI Educator of the year, both in 2023. He has received honoraria from Bausch + Lomb, CooperVision, Johnson & Johnson and Alcon.
1. Di Marco, A. Chow A. Acs M et al. Myopia in Practice (MIP) Study. American Academy Optom. 2023. Poster no 235334. 2. Wolffsohn JS, Whayeb Y, Logan NS, Weng R. IMI - global trends in myopia management attitudes and strategies in clinical practice - 2022 Update. Invest Ophthalmol Vis Sci. 2023;64(6):6. 3. Flitcroft DI, He M, Jonas JB, et al. IMI - Defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. 2019;60(3):M20-30. 4. Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447-68. 5. Chakraborty R, Read SA, Vincent SJ. Understanding Myopia: Pathogenesis and Mechanisms. In: Ang, M., Wong, T. (eds) Updates on Myopia. Springer, Singapore. 2019;65-94. 6. Smith Iii EL, Arumugam B, Hung LF, et al. Eccentricity-dependent effects of simultaneous competing defocus on emmetropization in infant rhesus monkeys. Vision Res. 2020;177:32-40. 7. Remington LA. Retina. In: Clinical Anatomy and Physiology of the Visual System (Third Edition), Butterworth-Heinemann. 2012;61-92.. 8. Wandell, BA. Foundations of Vision. Sinauer Associates, Sunderland, Mass. 1995 9. Ehsaei A, Chisholm CM, Pacey IE, Mallen EA. Off-axis partial coherence interferometry in myopes and emmetropes. Ophthalmic Physiol Opt. 2013;33(1):26-34. 10. Erdinest N, London N, Lavy I, ,et al. Peripheral defocus and myopia management: a mini-review. Korean J Ophthalmol. 2023;37(1):70-81. 11. Hiraoka T, Okamoto C, Ishii Y, et al. Contrast sensitivity function and ocular higher-order aberrations following overnight orthokeratology. Invest Ophthalmol Vis Sci. 2007;48(2):550-6. 12. Rozema JJ. Refractive development I: Biometric changes during emmetropisation. Ophthalmic Physiol Opt. 2023;43(3):347-67. 13. Yang J, Ouyang X, Fu H, et al. Advances in biomedical study of the myopia-related signaling pathways and mechanisms. Biomed Pharmacother. 2022;145:112472 14. Brown DM, Mazade R, Clarkson-Townsend D, et al. Candidate pathways for retina to scleral signaling in refractive eye growth. Exp Eye Res. 2022;219:109071. 15. Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci US A. 1989;86(2):704-6. 16. Tepelus TC, Schaeffel F. Individual set-point and gain of emmetropization in chickens. Vision Res. 2010;50(1):57-64. 17. Wildsoet CF, Chia A, Cho P, et al. IMI - Interventions Myopia Institute: Interventions for controling myopia onset and progression report. Invest Ophthalmol Vis Sci. 2019;60(3):M106-31. 18. Wolffsohn JS, Calossi A, Cho P, et al. Global trends in myopia management attitudes and strategies in clinical practice - 2019 Update. Cont Lens Anterior Eye. 2020;43(1):9-17. 19. Cherng-Hui Yip, V. Chen-Wei Pan, Xiao-Yu Lin, et al. The relationship between growth spurts and myopia in Singapore children. Invest Ophthalmol Vis Sci. 2012;53(13):7961-6. 20. Yii FS. Emmetropic eye growth in East Asians and non-East Asians. Ophthalmic Physiol Opt. 2023;43(6):1412-8. 21. Bullimore MA, Ritchey ER, Shah S, et al. The risks and benefits of myopia control. Ophthalmology. 2021;128(11):1561-79. 22. Cruickshank FE, Logan NS. Optical ‘dampening’ of the refractive error to axial length ratio: implications for outcome measures in myopia control studies. Ophthalmic Physiol Opt. 2018;38(3):290-7. 23. Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113:2285-91 24. Anhchuong Le, Bickol N. Mukesh, et al. Risk Factors Associated with the Incidence of Open-Angle Glaucoma: The Visual Impairment Project. Invest Ophthalmol Vis Sci. 2003;44(9):3783-9. 25. Tideman JWL, Polling JR, Vingerling JR, et al. Axial length growth and the risk of developing myopia in European children. Acta Ophthalmol. 2018;96(3):301-9. 26. Sanz Diez P, Yang LH, Lu MX, et al. Growth curves of myopia-related parameters to clinically monitor the refractive development in Chinese schoolchildren. Graefes Arch Clin Exp Ophthalmol. 2019;257(5):1045-53. 27. Lanca C, Pang CP, Grzybowski A. Effectiveness of myopia control interventions: A systematic review of 12 randomized control trials published between 2019 and 2021. Front Public Health. 2023;11:1125000. 28. Lam CSY, Tang WC, Zhang HY, et al. Long-term myopia control effect and safety in children wearing DIMS spectacle lenses for six years. Sci Rep 13, 5475 (2023). 29. Li X, Huang Y, Yin Z, et al. Myopia control efficacy of spectacle lenses with aspherical lenslets: results of a three-year follow-up study. Am J Ophthalmol. 2023;253:160-8. 30. Chalberg T, Laughton D, Hill J, et al. Control of Myopia Using Diffusion Optics Spectacle Lenses: Efficacy and Safety Study (CYPRESS) 42-month results. ARVO 2023 Annual Meeting. Invest Ophthlmol Vis Sci. 2023;64(5092). 31. Liu X, Wang P, Xie Z, et al. One-year myopia control efficacy of cylindrical annular refractive element spectacle lenses. Acta Ophthalmol. 2023;101(6):651-7. 32. Sánchez-Tena MÁ, Cleva JM, Villa-Collar C, et al. Effectiveness of a spectacle lens with a specific asymmetric myopic peripheral defocus: 12-month results in a Spanish population. Children (Basel). 2024;11(2):177. 33. Tarutta EP, Proskurina OV, Tarasova NA, et al. Long-term results of perifocal defocus spectacle lens correction in children with progressive myopia. Vestn Oftalmol. 2019;135(5):46-53. 34. Gregory, Hannah R. Nti, Augustine N. et al. Visual performance of center-distance multifocal contact lenses fit using a myopia control paradigm. Optom Vis Sci. 2021;98(3):272-9. 35. Singh S, Tian E. Comparing distance-center and near-center multifocal soft contact lenses for myopia control. Invest Ophthalmol Vis Sci. 2022;63(7):260-A0114. 36. Jones L, Drobe B, González-Méijome JM, et al. IMI - Industry guidelines and ethical considerations for myopia control report. Invest Ophthalmol Vis Sci. 2019;60(3):M161-83. 37. Cheng X, Xu J, Brennan NA. Randomized trial of soft contact lenses with novel ring focus for controlling myopia progression. Ophthalmol Sci. 2022;3(1):100232. 38. Díaz-Gómez S, Burgos-Martínez M, Sankaridurg P, et al. Two-year myopia management efficacy of extended depth of focus soft contact lenses (MYLO) in Caucasian children. Am J Ophthalmol. 2024;260:122-31. 39. Chamberlain P, Peixoto-de-Matos SC, Logan NS, et al. A three-year randomized clinical trial of MiSight lenses for myopia control. Optom Vis Sci. 2019;96(8):556-67. 40. https://reviewofmm.com/vti-shares-one-year-data-from-protect-trial-at-aao-2023. Tuan, KA. O’connor B. et al Poster GSLS 2024. 41. Sankaridurg P, Bakaraju RC, Naduvilath T, et al. Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: two-year results from a randomized clinical trial. Ophthalmic Physiol Opt. 2019;39(4):294-307. 42. Walline JJ, Walker MK, Mutti DO, et al. BLINK Study Group. Effect of high add power, medium add power or single-vision contact lenses on myopia progression in children: The BLINK Randomized Clinical Trial. JAMA. 2020;324(6):571-80. 43. Chia A, Chua WH, Cheung YB, et al. Atropine for the Treatment of Childhood Myopia: Safety and Efficacy of 0.5%, 0.1%, and 0.01% Doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119(2):347-354. 44. Li Y, Yip M, Ning Y, et al. Topical Atropine for childhood Myopia Control: The Atropine Treatment Long-Term Assessment Study. JAMA Ophthalmol. 2024;142(1):15-23. 45. Zhang XJ, Zhang Y, Yip BHK, et al. Five-Year Clinical Trial of the Low-Concentration Atropine for Myopia Progression (LAMP) Study: Phase 4 Report. Ophthalmology. 2024:S0161-6420(24)00190-8. 46. Klaver C, Polling JR; Erasmus Myopia Research Group. Myopia management in the Netherlands. Ophthalmic Physiol Opt. 2020;40(2):230-40. 47. Repka MX, Weise KK, Chandler DL, et al. Pediatric Eye Disease Investigator Group. Low-dose 0.01% Atropine eye drops vs. placebo for myopia control: a randomized clinical trial. JAMA Ophthalmol. 2023;141(8):756-65. 48. Ha A, Kim SJ, Shim SR, et al. Efficacy and safety of eight Atropine concentrations for myopia control in children: a network meta-analysis. Ophthalmology. 2022;129(3):322-3. 49. Jeon GS, Hong IH, Lee JH, et al. Analysis of treatment response about low-dose (0.01%) atropine eye-drops in myopic children. Eur J Ophthalmol. 2022;32(4):2011-7. 50. Zadnik K, Schulman E, Flitcroft I, et al. CHAMP Trial Group Investigators. Efficacy and safety of 0.01% and 0.02% Atropine for the treatment of pediatric myopia progression over three years: a randomized clinical trial. JAMA Ophthalmol. 2023;141(10):990-9. 51. Pucker A. Stating the facts about combination myopia management treatments. Optometry Times. https://www.optometrytimes.com/view/stating-the-facts-about-combination-myopia-management-treatments. May 24, 2023. Accessed May 26, 2024. 52. Nucci P, Lembo A, Schiavetti I, et al. A comparison of myopia control in European children and adolescents with defocus incorporated multiple segments (DIMS) spectacles, atropine and combined DIMS/atropine. PLoS One. 2023;18(2):e0281816. 53. Wang Z, Wang P, Jiang B, et al. The efficacy and safety of 0.01% atropine alone or combined with orthokeratology for children with myopia: A meta-analysis. PLoS One. 2023;18(7):e0282286. 54. Jones JH, Mutti DO, Jones-Jordan LA, Walline JJ. Effect of combining 0.01% Atropine with soft multifocal contact lenses on myopia progression in children. Optom Vis Sci. 2022;99(5):434-42. 55. Wang Q, Banerjee S, So C, et al. The effect of low-dose Atropine on alpha ganglion cell signaling in the mouse retina. Front Cell Neurosci. 2021;15:664491. 56. Yam J, Li FF, Zhang X, et al. Low concentration atropine for myopia progression (LAMP) Phase 3: efficacy of 0.05%, 0.025%, and 0.01% atropine eye drops over three years and the rebound phenomenon after cessation of treatment. ARVO 2020. Abstract 1133. |

