 |
The cornea is a unique, immune-privileged ocular structure that requires transparency for the individual to achieve optimal vision. Although it is normally avascular, it is still able to obtain adequate nourishment and efficiently undergo various cell processes, including mitosis and cellular healing/repair. These functions, along with the general integrity of the cornea, are made possible through the essential and adjacent area: the limbus.
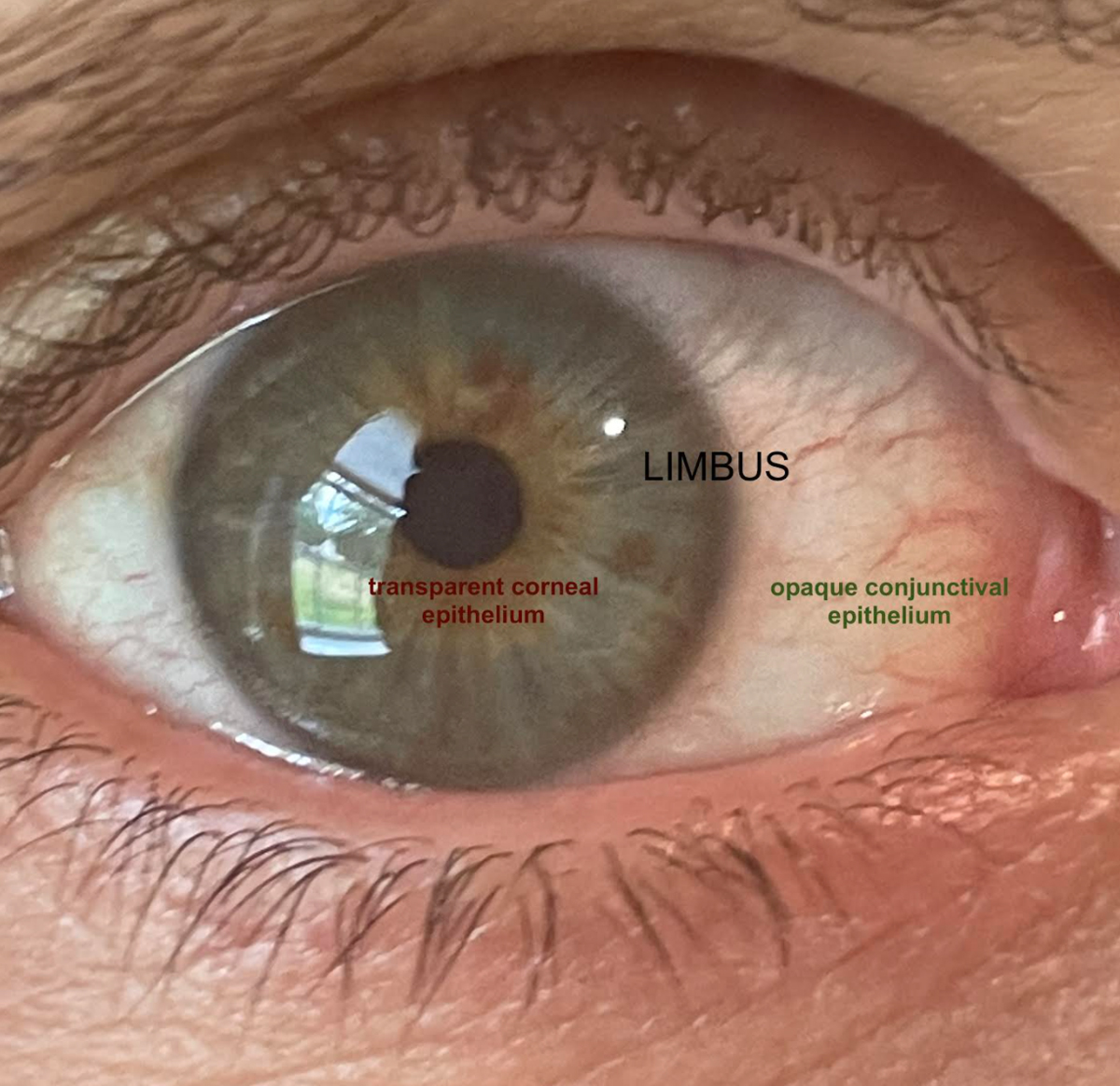
The limbus is defined as the transition zone between the opaque sclera and the clear cornea, separating the conjunctival epithelium and the corneal epithelium.1-3 The diameter is 1mm to 2mm wide and is often measured by the normal, gradual loss of transparency as the cornea extends toward the far periphery.1,2 This anatomical area in and of itself acts as a barrier prohibiting the invasion of conjunctival epithelial cells onto the cornea.3 It also, however, houses key components to corneal and ocular health, called limbal stem cells (LSCs).1-5
 |
|
The limbus is the transition zone preventing the opaque epithelium from invading the clear cornea. Click image to enlarge. |
Cells and Components
LSCs are most concentrated in areas of the limbus called the palisades of Vogt. To a lesser degree, they are also located in limbal epithelial crypts and the limbal epithelial pit. The limbal palisades of Vogt appear as fibrovascular ridges located radially and circumferentially around the peripheral cornea. These are readily viewed on the slit lamp under high magnification, appearing as a blue-gray ring encircling the periphery.1,2 The area of highest concentration of palisades is within the superior and inferior limbus. More specifically, these LSCs are located within the basal layer of the limbus and possess a higher degree of mitotic ability as compared with the peripheral and central cornea.4
The precise homeostasis of the LSC environment is critical in maintaining corneal integrity. It is what allows the cornea to remain transparent and avascular, to proliferate and heal and ultimately to provide the most optimal surface for light rays to be transmitted and focused on the retina for vision.4 A distinguishing feature of the cornea that would not be possible without the limbus is its avascularity. Unlike other ocular structures, the cornea is free of blood vessels, which allows for its transparency. The vasculature of the limbus, as well as the tear film, is what nourishes the cornea. The limbus is the zone that prevents the opaque conjunctival epithelium from invading the otherwise clear cornea.1-5
Damage and Disease
When dysfunction arises, as in various pathological conditions, the environment is disrupted. When the limbus, which is rich in stem cells, is injured, this leads to cellular apoptosis. LSCs typically differentiate into corneal epithelial cells, but when they are injured, they are repaired and filled in by the conjunctival epithelium. There is a stark difference between these two types of epithelia, as the conjunctiva contains goblet cells and is highly vascularized. This ultimately leads to blood vessels invading the cornea, which is called neovascularization.
Evidence of neovascularization is a clinical indication that key components of the limbus, the LSCs, are damaged.4,5 Additionally, invasion of the conjunctival epithelium onto the corneal surface leads to an irregular ocular surface, diminished tensile strength and inept barrier function.5
Generally speaking, corneal neovascularization affects up to 1.4 million people per year and is a common cause of vision loss.5 Growth of new blood vessels can occur in various regions of the cornea, including the epithelium and the stroma. Because of the limbal vascularity and the avascularity of the cornea, neovascularization begins peripherally and can progress to the central cornea, leading to opacification and vision loss. Being able to accurately identify and determine the underlying cause of corneal neovascularization is key, as many treatment options are most effective only in the early stages.3
Table 1. Overview of LSC Damage | |
| Staging of LSC Deficiency | Clinical Findings |
| Stage 1: Mild | Dull corneal reflex Epithelial thinning and pooling |
| Stage 2: Moderate | Vortex keratopathy/whorl-like epitheliopathy |
| Stage 3: Severe | Corneal melt and/or perforation Stromal scarring and neovascularization |
Cause and Chronicity
LSC deficiency is a gradual process and can occur from primary or secondary causes. Using clinical clues, like neovascularization, is necessary in identifying this dysfunction and preventing vision loss as early and effectively as possible. Primary causes are characterized by genetic mutations that are often congenital and directly affect the integrity or function of LSCs. Such cases include aniridia, congenital epidermal dysplasia, Turner syndrome, keratitis secondary to endocrine deficiencies and xeroderma pigmentosum.
The other category of LSC deficiency is comprised of secondary conditions that cause damage to LSCs due to external conditions. Most commonly, these include thermal or chemical injury, chronic inflammation, injury from ocular surgeries, contact lens wear, infection, keratopathies and ocular surface tumors.3
Diagnosis of LSC deficiency is made through clinical examination. Symptoms are often nonspecific and may be mild in the early stages, such as conjunctival redness, foreign body sensation, photophobia and tearing. As LSC deficiency advances, delayed epithelial wound healing is evident through symptoms of pain and decreased vision. Clinically, this would manifest as recurrent corneal erosions.3
There are three stages of LSC deficiency that depend on clinical clues and examination with slit lamp biomicroscopy. While examination with white light is routinely performed, diagnosing LSC deficiency is easier through the use of fluorescein under cobalt blue light.3
Mild stage. The first and earliest stage of LSC deficiency is denoted by a dull, irregular corneal reflex. This is due to the combination of conjunctival and corneal epithelial cells, inhibiting optimal transparency of the cornea. Additionally, there may be areas of thinning and pooling of fluorescein because the abnormal epithelium is thinner and lacks tight junctions. Neovascularization is usually not present in the milder stage, but there may be areas of pannus peripherally, outside the central 5mm of the cornea. There is also less than 50% limbal involvement. To identify loss of the palisades of Vogt, careful examination of LSC location, especially inferiorly and superiorly, is necessary. The limbus may also appear more flat than healthy eyes.3,4
Moderate stage. As LSC deficiency advances, the corneal epithelium may take on a spiral pattern similar to vortex or whorl-like keratopathy. This area is susceptible to epithelial erosions. Neovascularization or pannus may be seen centrally, and over 50% of the limbus is involved in this stage.3,4
Severe stage. The third and final stage is the most severe display of LSC deficiency, resulting in corneal melt or perforation from the chronic, poor epithelial wound healing. Opacification takes over the entire cornea, and deeper stromal neovascularization and scarring are often present. Total blindness is a potential and devastating outcome of this condition.3,4
Treatment is most effective in the early stages of LSC deficiency. As such, a timely and accurate diagnosis is vital for the best visual outcome. Treatment is also variable and related to the underlying etiology. While we routinely examine both the conjunctiva and the cornea, we need to pay closer attention to the important transitory zone of the limbus, which houses the most important cells for corneal function.
Dr. Labib graduated from Pennsylvania College of Optometry, where she now works as an associate professor. She completed her residency in primary care/ocular disease and is a fellow of the American Academy of Optometry and a diplomate in the Comprehensive Eye Care section. She has no financial interests to disclose.
1. Peraza Nieves J, Rocha de Lossada C, Sabater Cruz N, Torras Sanvicens J. Human corneo-conjunctival limbus anatomy assessed by scanning electron microscopy. Indian J Ophthalmol. 2020;68(8):1665. 2. Bergmanson JP, Martinez JG. Size does matter: what is the corneo-limbal diameter? Clin Exp Optom. 2017;100(5):522-8. 3. Le Q, Xu J, Deng SX. The diagnosis of limbal stem cell deficiency. Ocul Surf. 2018;16(1):58-69. 4. Ruan Y, Jiang S, Musayeva A, et al. Corneal epithelial stem cells-physiology, pathophysiology and therapeutic options. Cells. 2021;10(9):2302. 5. Sharif Z, Sharif W. Corneal neovascularization: updates on pathophysiology, investigations & management. Rom J Ophthalmol. 2019;63(1):15-22. |

