 A 60-year-old white male returned to the clinic with a chief complaint of visual disturbances in his right eye. His ocular and medical histories were unremarkable.
A 60-year-old white male returned to the clinic with a chief complaint of visual disturbances in his right eye. His ocular and medical histories were unremarkable.
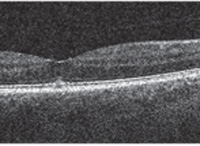
Best-corrected visual acuity at distance measured 20/25 OU. Slit lamp evaluation was unremarkable, revealing only very mild brunescence of both crystalline lenses. With the exception of positive metamorphopsia in his right eye, all preliminary testing was within normal limits OU. Dilated fundus examination revealed a small, cyst-like elevation OD. Spectral-domain optical coherence tomography (SD-OCT) confirmed the presence of vitreomacular traction OD that was associated with a small vitreomacular base attachment (figure 1).
 |
|
| 1. Would Jetrea be appropriate for this
vitreomacular traction patient? |
What is VMA?
Vitreous liquefaction results in the separation of the posterior vitreous cortex from the superficial retina. The resultant posterior vitreous detachment (PVD) may remain partially attached to the macula. In turn, residual attachment may lead to vitreomacular adhesion (VMA), which is associated with several maculopathies, including tears and holes. Vitreomacular adhesion is characterized as an incomplete detachment of the posterior vitreous hyaloid with persistent adherence to the macula.1 The natural course of VMA is unpredictable. Some cases self-resolve, while others progress and eventually cause further structural damage. Many patients who present with VMA exhibit visual acuity disruption or vision loss. The management of VMA typically is limited to close observation or surgery. Pars plana vitrectomy (PPV) still is regarded as the conventional treatment approach for patients with VMA. Although PPV is associated with resolution of the localized vitreomacular attachment, the procedure may yield numerous adverse effects, including cataract formation, infections and retinal detachment. Thus, PPV typically is reserved for individuals with progressive VMA or those with associated vision loss––which leaves a number of symptomatic patients with unmet needs.Jetrea: An Alternative to PPV
In October 2012, Jetrea received FDA approval for the treatment of symptomatic VMA. The injection is comprised of microplasmin, an active protease derived from plasmin, which induces vitreous liquefaction and subsequent lysing of the posterior vitreous cortex from the vitreoretinal interface.2,3Jetrea’s approval was secured upon publication of clinical data from the Microplasmin for IntraVitreous Injection-Traction Release without Surgical Treatment (MIVI-TRUST) study.4-6 More than 600 subjects with VMA participated in the MIVI-TRUST study. Approximately two-thirds of the patients received a 125µg intravitreal microplasmin injection, and the remaining participants received a sham injection. The study results indicated that 26.5% of the treated subjects exhibited VMA resolution, which was associated with a complete PVD. By comparison, just 10.1% of the sham treatment group achieved a complete PVD.6
Further, twice as many patients in the treatment arm experienced improved visual acuity than those in the placebo group.Additionally, the MIVI-TRUST study had an excellent overall safety record. Patients enrolled in the treatment group experienced only mild adverse effects, associated with localized injections, including floaters, photopsia and eye pain.6
A Forecast for Success?
Since its approval, Jetrea has been shown to effectively resolve VMA associated with a complete PVD. A recent publication described the initial clinical experience in a single eye center.6 In this retrospective review, researchers administered intravitreal injections of Jetrea to 19 patients who exhibited a variety of clinical presentations secondary to VMA. The patients’ ages ranged from 57 to 81 years. After a mean follow-up of 56 days, approximately 40% of patients demonstrated alleviation of the vitreoretinal traction, as well as a modest overall improvement in visual acuity.6 Additionally, half of the patients who initially presented with an associated macular hole experienced complete closure following Jetrea injection.6 It is important to reiterate that the aforementioned study was limited to just 19 patients. Other studies have indicated that baseline features can predict the success of Jetrea injection.7,8 For example, a retrospective analysis of patients who participated in the MIVI-TRUST study was presented at the 2012 Annual Meeting of the American Academy of Ophthalmology in Chicago.8More than 460 patients treated with Jetrea were evaluated. The positive independent baseline features related to VMA resolution included anatomical features, such as phakia, smaller VMA attachment diameter, presence of concomitant macular hole and absence of epiretinal membrane, as well as individual factors, such as age.8
Optimized patient outcomes following Jetrea injection may require a thorough clinical evaluation for pertinent baseline findings. Only then will you have a reasonable idea whether an individual’s prognosis for VMA resolution and subsequent visual recovery is favorable.
Dr. Shechtman is a member of Thrombogenics’ optometric advisory board. Neither she nor Dr. Karpecki have direct financial interest in any of the products mentioned.
1. Smiddy WE, Green WR, Michels RG, de la Cruz Z. Ultrastructural studies of vitreomacular traction syndrome. Am J Ophthalmol. 1989 Feb 15;107(2):177-85.2. Stalmans P. A randomized, double-masked, clinical trial of microplasmin intravitreal injection for nonsurgical treatment of vitreomacular traction. Presented at the Annual Meeting of the American Society of Retinal Specialists. Palm Spring, Calif. December 2007.3. Gandorfer A, Rohledder M, Sethi C, et al. Posterior vitreous detachment induced by microplasmin. Invest Ophthalmol Vis Sci. 2004 Feb;45(2):641-7. 4. Jumper J, Pakola S. The MIVI-007 trial. Phase III evaluation of single intravitreous injection of microplasmin or placebo for treatment of focal vitreomacular adhesion. Paper presented at the American Society of Retina Specialists; August 31, 2010; Vancouver, BC.5. Packo K, Pakola S. The MIVI-006 trial. Phase III evaluation of single intravitreous injection of microplasmin or placebo for treatment of focal vitreomacular adhesion. Paper presented at the Annual Meeting of the American Society of Retina Specialists. Vancouver, BC. August, 2010.6. Stalmans P, Benz MS, Gandorfer A. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012 Aug 16;367(7):606-15.7. Kim BT. Initial outcome following intravitreal ocriplasmin for treatment of symptomatic vitreomacular adhesion. Ophthalmic Surg Lasers Imaging Retina. 2013 Jul-Aug;44(4):334-43.8. Ray S. Baseline anatomic features predictive of pharmacologic vitreomacular adhesion resolution in the phase III ocriplasmin clinical trial program. Presented at the American Academy of Ophthalmology Retinal Subspecialty Day. Chicago. November, 2012.

