 A 39-year-old white female presented with blurred vision in her left eye that had persisted for four days. She reported that her central vision was unaffected; but, she noticed some visual distortion just outside of her central vision.
A 39-year-old white female presented with blurred vision in her left eye that had persisted for four days. She reported that her central vision was unaffected; but, she noticed some visual distortion just outside of her central vision.
She reported having a comprehensive eye examination two months earlier. At that time, her eyes were healthy, and she simply required an adjustment in her optical correction. Her ocular history was significant for high myopia, which she manages with daily-wear gas-permeable contact lenses. The patient was in excellent health and took no medications.
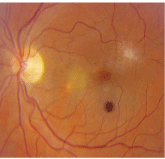
1. Posterior pole image of our patients left eye. Note the changes both superotemporal and inferonasal to the fovea. The dark lesion located below the fovea is an artifact.
On examination, her best-corrected visual acuity measured 20/20 O.D. and 20/25 O.S. Her refractive error was -10.50D-1.25D x 170 O.D. and -11.00D-2.00D x 005 O.S. Extraocular motility testing was normal. Confrontation visual fields were full to careful finger counting O.U. Her pupils were equally round and reactive, with no afferent pupillary defect.
Amsler grid testing was normal O.D. She had metamorphopsia at the edge of the grid superotemporally O.S. Her anterior segment exam was unremarkable O.U.
Dilated fundus exam showed small cups with good rim coloration and perfusion O.U. The vessels, macula and periphery were normal O.D. However, her left eye exhibited an area of subretinal fluid inferonasal to the macula and some peculiar changes superiorly (figure 1). Additionally, we performed a spectral domain optical coherence tomography (SD-OCT) scan (figure 2).
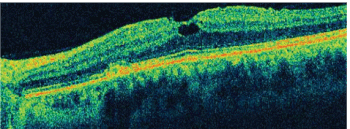
2. A spectral-domain optical coherence tomography (SD-OCT) image through the left macula of our patient. What do you notice?

Take the Retina Quiz
1. What does the lesion located superior temporal to the macula O.S. represent?
a. Epiretinal membrane (ERM).
b. Exudate.
c. Glial blastoma.
d. Choroidal rupture.
2. Based on the SD-OCT image, how would you characterize the fovea O.S.?
a. Healthy and normal.
b. Stage I macular hole.
c. Pseudohole.
d. Early lamellar hole.
3. What does the SD-OCT scan reveal nasal to the macula O.S.?
a. Neurosensory detachment.
b. Pigment epithelial detachment (PED) with focal areas of serous retinal detachment.
c. Cystoid macular edema (CME).
d. Epiretinal membrane.
4. What is the correct diagnosis in this case?
a. Macular edema secondary to an ERM.
b. Central serous retinal detachment.
c. Choroidal neovascularization (CNV) secondary to degenerative myopia.
d. Stage I macular hole.
5. How should this patient be treated?
a. Observation.
b. Thermal laser.
c. Photodynamic therapy.
d. Avastin (bevacizumab, Genentech) injection.
For answers, see below.
Discussion
Our patients left macula exhibits some interesting features. The white, glistening focal lesion seen superiorly represents an ERM that has caused some disruption of her macula. After reviewing the SD-OCT, it appears that she may have an early lamellar hole as a result of the ERM.
We have no definitive explanation why our patient has the ERM. Most often, ERMs occur in elderly patients as a result of a complicated posterior vitreous detachment (PVD) or previous retinal surgery. Because our patient neither experienced a PVD nor underwent retinal surgery, we assume that the ERM is either idiopathic or related to her high myopia. Fortunately, the ERM is not the cause of her acute visual change.
Indeed, her visual symptoms are caused by the localized area of subretinal fluid inferiornasal to the fovea. On clinical examination, an area of retinal whitening is present that likely represents de-pigmentation of the retinal pigment epithelium (RPE). In this area on the SD-OCT, subretinal fluid and some small areas of RPE detachment are apparent. Some areas of high reflectivity that likely represent CNV are also evident on SD-OCT. The CNV developed from the area of RPE depigmentation as a result of her degenerative myopia.
Myopia is classified as simple, congenital or degenerative. Degenerative myopia constitutes refractive error greater than -6.00D and an axial length greater than 26mm.1
The clinical features of degenerative myopia include peripapillary atrophy, tilted discs, posterior staphyloma, RPE thinning and atrophy, lacquer cracks (which result from rupture of Bruchs membrane) and Fuchs spots. More recently, macular schisis has been seen in degenerative myopia and is thought to be a precursor to macular hole formation.2
Degenerative myopia is the leading cause of blindness in the world and is the second most common cause of CNV behind age-related macular degeneration.1 CNV occurs in approximately 5% to 10% of myopes with axial lengths greater than 26.5mm.1 New blood vessels typically grow through breaks or defects in Bruchs membrane, or, in some cases, they may develop as a result of an atrophic area within the RPE. Clinically, the CNV may appear as a round or elliptical light gray lesion at or near the fovea. As the CNV matures, subretinal hemorrhage and exudation may occur. Our patient demonstrated serous elevation of the sensory retina and RPE, but no subretinal hemorrhages or exudates were evident.
Fluorescein angiography (FA) is very sensitive in identifying the size and location of CNV. In this case, however, FA was unnecessary.
The treatment of CNV associated with degenerative myopia has been difficult and somewhat controversial. In the past, thermal laser was employed, which demonstrated varying degrees of success. Because of the thin, brittle nature of Bruchs membrane and the RPE, postoperative scarring often results in suboptimal visual outcomes.
Also, thermal laser frequently permits recurrences of CNV, necessitating retreatment procedures. Photodynamic therapy has also been used, but it too yields indefinite visual success and may allow for a high recurrence rate of CNV.3 Today, intravitreal anti-vascular endothelial growth factor agents hopefully will offer better visual outcomes for patients with CNV.
Our patient received an intravitreal injection of Avastin (bevacizumab, Genentech) at her initial visit. One month later, she demonstrated complete resolution of the area of subretinal fluid. She showed no recurrences of CNV at several subsequent follow-up examinations, and her vision remained stable at 20/25 O.S. (figure 3).
3. An SD-OCT image of our patients left eye five months after treatment.
1. Soubrane G, Coscas GJ. Choroidal neovascular membrane in degenerative myopia. In: Ryan SJ (ed). Retina, Vol. II: Medical Retina, 4th ed.
2. Takano and S. Kishi. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol 1999 Oct;128(4):472-6.
3. Blinder KJ, Blumenkranz MS,
Retina Quiz Answers: 1) a; 2) d; 3) b; 4) c; 5) d.

