At that time, the doctor mentioned current literature that indicated that patients like her were at minimal risk of developing glaucoma. The doctor recommended she discontinue her Xalatan and return in 12 months for follow up. She was uneasy about this abrupt change in her therapeutic course and sought the second opinion a few months after she discontinued her medication.
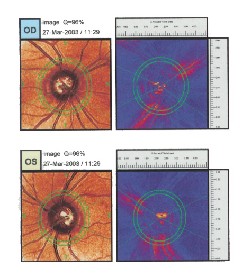 |
| Scanning laser polarimetry indicates thin areas of the perioptic nerve fiber layer. |
Diagnostic Data
Best-corrected visual acuity was 20/20 O.U. through low hyperopic, astigmatic and presbyopic correction. Pupils were equal, round and reactive to light and accommodation with no afferent pupillary defect. Extraocular motilities were full O.U.
A slit lamp examination of her anterior segments was completely unremarkable. Applanation tensions were 27mm Hg O.D. and 26mm Hg O.S. CCT readings were 598m O.D. and 589m O.S. Gonioscopic examination of the anterior chamber angles demonstrated grade III open angles with moderate trabecular pigmentation O.U.; there were no angle abnormalities.
Through dilated pupils, her crystalline lenses were clear O.U. Her cup-to-disc ratios were 0.75 x 0.75 O.D. and 0.60 x 0.65 O.S., with the thinnest regions of her neuroretinal rims being 8-11 oclock O.D. and 1-4 oclock O.S. This correlated well to thinned areas of the perioptic nerve fiber layer as measured by polarimetry. Her macular and peripheral retinal evaluations were normal.
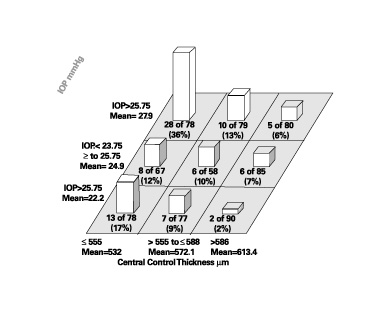 |
| The patient in this case had IOPs of 27mm Hg O.D. and 26mm Hg O.S., and CCT readings of 598m O.D. and 589m O.S. These measurements indicate a relatively low likelihood of developing glaucoma when compared against the results from the Ocular Hypertension Treatment Study.1 |
Following my evaluation of this patient, we discussed the clinical findings at length. It appeared as though she carried a diagnosis of elevated IOP for some time, and the previous doctor initially felt she had glaucoma. But she indicated that because of new literature, and its reference to the thickness of something, her previous doctor felt it would be appropriate to discontinue medication. But her uneasiness about that choice brought her in for the second opinion.
As best as I can tell, her previous doctor probably based the change in therapy on the findings associated with CCT measurements and their relationship with the probability of developing glaucoma, most notably as a result of the Ocular Hypertension Treatment Study (OHTS).1 According to OHTS, this patients CCTs and IOPs would indicate a relatively low likelihood of developing glaucoma. Perhaps the doctor felt she was an ocular hypertensive patient rather than a frank glaucoma patient and decided to monitor her as opposed to actively intervening with therapy.
Diagnosis and Treatment
I felt that the patient did in fact have glaucoma and that she would be best served on therapy. I based my conclusions on several issuesfirst and foremost the contours and anatomy of her neuroretinal rim. This was also objectively supported by scanning laser polarimetry. And though her visual fields did not show significant field loss, pre-perimetric glaucoma is a clinical entity that does exist. She has a family history of glaucoma, and her cupping did not correlate well with the typical hyperopic disc appearance.
I chose to medicate her with Travatan (travoprost, Alcon), 1 drop O.U. h.s.
On subsequent evaluation, her IOPs are averaging 14mm Hg O.D. and O.S. Her neuroretinal rims and visual fields have remained stable.
Discussion
While it is important to be familiar with current research, we must never lose sight of the overall clinical picture of the patient. And we must make sure we do not take snippets of information from these studies and apply them in clinical practice without relating that information to the patient at hand, and without relying on evidence-based research and clinical intuition.
When I began practice several years ago, making therapeutic decisions in glaucoma care based on one isolated finding, such as IOP, was not uncommon. Although we now see that this cookbook approach is inadequate, we need to be careful that we dont find ourselves doing the same thing in a different context, such as basing a therapeutic decision solely on CCT measurements. While CCT and its relationship to glaucoma is a relatively new parameter that must be evaluated, remember that CCT is only a risk factor in the development of glaucoma, and must be evaluated in the context of the entire glaucomatous landscape. Failure to do so, as in this case, has the clinician coming full circle to employing bad habits (basing clinical decision on one finding, i.e. CCT) that we used to do all too commonly (like treating based solely on elevated IOP).
Am I saying we should not reassess our patient management based on new clinical data, such as CCT? Absolutely not. A recent study looked at just this issue.
In this study, researchers looked at how CCT findings would alter the treatment plans already established for patients who had glaucoma and glaucoma suspect diagnoses.2 Treatment plans could either be increased (begin therapy, increase medications, proceed to laser or proceed to filtering) or decreased (cessation of therapy, decrease medication or cancellation of laser or filtering procedures) based upon CCT-adjusted IOP measurements. While the study did in fact show that changes in management are appropriate and clinically significant in some situations, the majority (about 87%) of individuals enrolled in the retrospective study did not undergo therapy modification.
What is the entire glaucomatous landscape that I refer to? Another study concludes that the global risk of progression incorporates all available data, though it is an evolving process.3 So, as with any evolving process, as we learn more about glaucoma, we must be prepared to change tack when needed, but also stay the course when needed.
- Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002 Jun;120(6):714-20.
- Shih CY, Graff Zivin JS, Trokel SL, Tsai JC. Clinical significance of central corneal thickness in the management of glaucoma. Arch Ophthalmol 2004 Sep;122(9):1270-5.
- Weinreb RN, Friedman DS, Fechtner RD, et al. Risk assessment in the management of patients with ocular hypertension. Am J Ophthalmol 2004 Sep;138(3):458-67.

