 As eye care practitioners, we are professionally charged with maintaining the ocular health and vision of our patients. Periodically, however, we encounter a patient with a blind eye. When vision is totally lost, we sometimes believe that our role is unnecessary. But, that may not be the case.
As eye care practitioners, we are professionally charged with maintaining the ocular health and vision of our patients. Periodically, however, we encounter a patient with a blind eye. When vision is totally lost, we sometimes believe that our role is unnecessary. But, that may not be the case.
Here, we’ll discuss potential management options for two patients who presented with painful, blind eyes.
Patient One
The first patient was a 33-year-old female with near total hearing loss and mild intellectual disability. Her ocular history was significant for blunt trauma to her right eye caused by a rock many years earlier. She received basic first aid at the time, but nothing more. Her family reported that she had very poor vision in that eye––but it was difficult to assess, given her cognitive impairment.
She presented with a totally blind right eye, which––according to her family––had been causing intermittent pain for many months. (They were unsure of the pain level because the patient tolerated discomfort well and did not express herself completely.) Her right cornea was edematous, with microcystic edema and bullous keratopathy. The anterior chamber was free of cells and flare.
The intraocular pressure (IOP) in that eye measured 72mm Hg, and there was total glaucomatous atrophy of the optic disc. Gonioscopic evaluation revealed a total angle recession.
When confronted with the diagnosis and educated about the condition, the patient’s family expressed a desire for the patient to be pain free and exhibit a cosmetically normal eye.
Patient Two
The second patient was a 52-year-old female with a longstanding history of blunt trauma in her left eye. Like patient one, she presented with a painful, totally amaurotic eye. She had significant angle recession in that eye as well as a hypermature lens, which displaced laterally behind the iris, leaving her functionally aphakic.
She had a mild anterior chamber reaction, likely due to phacolysis. She also had a left constant exotropia, presumably from the longstanding visual deprivation. She exhibited microcystic edema and bullous keratopathy, which accounted for her pain. Further, her IOP measured 55mm Hg OS.
Previously, she had used multiple glaucoma medications and reported that, when she stopped using the medications, her eye became painful. The patient was concerned that she spent too much money treating a blind eye and wanted to explore other options.
What to Do?
It is a common question that we get from students and residents: “Do you treat a blind eye?” As there may be no overall consensus that covers every situation, we approach each case individually. However, we have found that there are many instances when patients do benefit from therapeutic intervention in a blind eye. So, the short answer to this question: “Yes, you do treat a blind eye.”
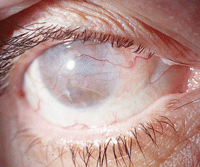
Even in a blind eye, as seen here, therapeutic intervention may be necessary to control pain or improve appearance.
There are numerous causes of painful, blind eyes, including glaucoma, neovascular disease, chronic retinal detachment with resultant ischemia, ocular ischemic syndrome and intraocular tumors, to name a few. Probably one of the most commonly encountered causes of painful, blind eyes is uncontrolled glaucoma with extremely elevated IOP.
In chronic glaucoma, IOP rises slowly for many years. When this happens, the eye typically autoregulates and adapts to the elevated IOP from a corneal perspective, while the optic nerve suffers. However, after prolonged periods of elevated IOP (typically exceeding 50mm Hg), the eye loses its ability to compensate, and the endothelial sodium-potassium pumps––charged with preventing aqueous from breaching and reaching the stroma––begin to malfunction.
The result is aqueous seeping through the endothelium into the stroma, causing corneal edema. This results in a slightly cloudy cornea. If the eye has vision, the patient will complain of halos around lights at night, because light is diffracted differently through the edematous cornea. As the edema accumulates, the microcysts can form larger bullae, which may migrate to the corneal surface, rupture and cause significant pain.
Over time, the rupturing bullae, as well as the persistent edema, create a neurotrophic environment. Then, the corneal nerves––which are responsible for reflex tearing––deaden. The cornea becomes dry and atrophic, and scar tissue and blood vessels replace the normally clear stroma.
Based upon this vicious cascade, it is evident that IOP needs to be reduced––even in a blind eye––to prevent the development of corneal edema, bullae (with resultant pain) and corneal scarring.
Treatment Options
In these situations, heroic treatment measures are not necessary. A blind eye does not require IOP as low as a sighted eye with glaucoma. It seems that an IOP less than 40mm Hg is sufficient to prevent the aforementioned cascade from occurring.
• Topical therapy. Any of the common glaucoma medications can be used sparingly––provided that there are no systemic contraindications––in order to reach an IOP less than 40mm Hg. If there is any degree of pain or inflammation present, a cycloplegic (e.g., atropine) and steroid (e.g., prednisolone) can be given once or twice per day. These measures may be employed indefinitely. Always remember to use the least amount of medication possible to achieve a painless, cosmetically acceptable eye.
• Traditional surgical intervention. If topical therapy cannot control IOP, there are more invasive options. Traditional glaucoma surgery, such as trabeculectomy, will not be performed on a sightless eye because surgeons cannot justify the procedure when there is no visual potential.
In cases of severe glaucoma that does not respond to topical therapy, cyclodestructive procedures can be employed.1-3 These techniques destroy the secretory neuroepithelium of the ciliary body. By doing so, aqueous production is diminished and IOP is reduced. The secretory neuroepithelium of the ciliary body can regenerate, however, necessitating multiple procures in some cases.
Older procedures have accomplished this with either cryotherapy or Nd:YAG photodestruction—the energy used to debilitate the ciliary body is applied externally across the sclera.
The advantages are that the globe isn’t opened and the procedure can be performed in the office. The primary disadvantage is that more energy is used to achieve the aim, creating more inflammation and possibly worsening the situation. Further, these procedures increase the risk of postoperative uveitis, hyphema, IOP spike, ciliary-block glaucoma, hypotony and phthisis bulbi.
• Advanced surgical intervention. A modern approach is to employ endoscopic diode laser cyclodestruction. This usually is done while the globe is open during cataract surgery on patients with concurrent glaucoma. However, this procedure is performed only in eyes with visual potential, and would not be done in a sightless eye (where the older techniques are more likely to be employed).
Another option is a neurolytic block via retrobulbar alcohol injection. The alcohol infiltrates the long and short posterior ciliary nerves, causing coagulation and protein/lipid precipitation.4 Although this doesn’t control IOP, the pain diminishes from destruction of these sensory nerves.
If the nerves are not completely destroyed, pain can remain and will correlate with the amount of neural preservation. Keep in mind that these nerves can partially regenerate, and pain may return after the procedure. Repeat injections may be necessary, but pain relief can last approximately two years.
More recently, chlorpromazine has been used in this fashion rather than alcohol. The action appears to be cell lysis resulting in membrane stabilization of the ciliary ganglion. There is a higher success rate of pain control with chlorpromazine compared to alcohol, with effective pain relief for an average of 12.5 months.5
Additionally, retrobulbar chlorpromazine may decrease IOP through an unknown mechanism. There are numerous potential complications with retrobulbar blocks, however, including ophthalmoplegia and retrobulbar hemorrhage.
• Enucleation and evisceration. The most extreme options to address a painful, blind eye are enucleation and evisceration.6
Enucleation usually is reserved for patients with severe ocular disfigurement, phthisical eyes, orbital tumors or when other methods of pain control have failed. The procedure involves removal of the entire globe, with only the extraocular muscles remaining. Pain relief is achieved within six months of globe removal. A cosmetically acceptable prosthesis is then inserted.
Evisceration is the complete removal of the inner contents of the eye, leaving only the scleral shell and extraocular muscles. The advantage of evisceration over enucleation is a faster recovery, less damage to orbital tissues, and better cosmesis and prosthesis movement. Additionally, pain relief is achieved within six weeks to six months following treatment. After removal of the orbital contents, an inert, porous implant is placed within the scleral shell to fill the volume of the orbit.
We prescribed numerous glaucoma medications for our first patient. Despite maximum medical therapy, her IOP never fell below 40mm Hg OD. We decided that she should undergo cyclodestruction to control pain and reduce medication dependency. Following two cyclodestructive procedures, her IOP still did not drop below 40mm Hg. However, she was maintained in a pain-free state with the use of atropine and prednisolone 1.0% QD and Combigan (brimonidine tartrate/timolol maleate) BID.
Unfortunately, the chronic IOP over 40mm Hg led to corneal scarring and opacification. Despite the cosmetic appearance, the patient’s family is happy that she is pain free, and wishes to avoid enucleation and prosthesis use for as long as possible.
The second patient elected to undergo a retrobulbar alcohol injection. She tolerated the procedure well and remained pain free for seven months. Her IOP remained unchanged and, following a period of pain relief, she decompensated.
We educated her about the potential benefits and limitations of repeat injection and enucleation, but she declined any further surgical intervention.
She was then successfully managed with atropine and prednisolone acetate 1% QD and dorzolamide hydrochloride/timolol maleate BID.
Thanks to Olena Moiseiykina, OD, of Davie, Fla., for inspiring this month’s column.
1. Pastor SA, Singh K, Lee DA, et al. Cyclophotocoagulation: a report by the American Academy of Ophthalmology. Ophthalmology. 2001 Nov;108(11):2130-8.
2. Schlote T, Derse M, Rassmann K, et al. Efficacy and safety of contact transscleral diode laser cyclophotocoagulation for advanced glaucoma. J Glaucoma. 2001 Aug;10(4):294-301.
3. Walland MJ. Diode laser cyclophotocoagulation: Dose-standardized therapy in end-stage glaucoma. Aust N Z J Ophthalmol. 1998 May;26(2):135-9.
4. Al-Faran MF, Al-Omar OM. Retrobulbar alcohol injection in blind painful eyes. Ann Ophthalmol. 1990 Dec;22(12):460-2.
5. Chen TC, Ahn Yuen SJ, Sangalang MA, et al. Retrobulbar chlorpromazine injections for the management of blind and seeing painful eyes. J Glaucoma. 2002 Jun;11(3):209-13.
6. Migliori ME. Enucleation versus evisceration. Curr Opin Ophthalmol. 2002 Oct;13(5):298-302.

