 |
| Fig. 1. This patient presented with dacryocystitis. Click image to enlarge. Photo: Christine Sindt, OD |
In light of the evidence, we will cover strategies and information to empower clinicians with the resources and information they need to make sound decisions pertaining to antibiotic use.
 |
| Fig. 2. This patient presented with an instance of cellulitis. Photo: Christine Sindt, OD |
First, Know Thine Enemy
In adults, several well-recognized gram-positive microbes comprise the list of the most common pathogens—including several staphylococcal species. They range from the opportunistic pathogen Staphylococcus epidermidis, an organism that commonly colonizes the ocular adnexa as a normal part of the ocular flora, up to the true pathogen Staphylococcus aureus, which produces exotoxins that allow it to produce significant tissue damage and threaten sight. Regarding resistance, the most problematic member of the staphylococcal family of pathogens is MRSA—methicillin-resistant S. aureus.3Other gram-positive organisms include several Streptococcus species. For example, Streptococcus pneumonia can be particularly virulent. It produces enzymes including streptokinase and hyaluronidase that allow it to penetrate tissue, potentially leading to corneal perforation or orbital cellulitis.3
Common gram-negative pathogens include Haemophilus species, Pseudomonas and Neisseria. See Table 1 for the full list of the common ocular pathogens.
Table 1. Common Ocular Pathogens3 |
| I. Gram-positive Organisms A. Staphylococci 1. S. aureus 2. S. epidermidis B. Streptococci 1. alpha-Hemolytic streptococcus 2. beta-Hemolytic streptococcus 3. Streptococcus pneumoniae C. Bacilli (rods) 1. Bacillus a. B. anthracis b. B. cereus c. B. subtilis 2. Cornybacterium a. C. diphtheria b. C. xerosis 3. Listeria 4. Nocardia 5. Mycobacterium II. Gram-negative Organisms A. Neisseria 1. N. gonorrhea 2. N. meningitidis B. Bacilli 1. Enterobacteriaceae a. E. coli b. Shigella c. Klebsiella d. Serratia e. Proteus 2. Moraxella 3. Haemophilus a. H. Influenza b. H. aegyptius 4. Brucella 5. Pseudomonas a. P. aeruginosa b. P. cepacia |
Betting the Farm
The worldwide animal industry is estimated to use more tons of antibiotics than does all of human medicine, a practice that elevates the risk of treatment failure for us all.4 For the growing antibiotic resistance problem to be effectively contained or reversed, responsible antibiotic use in the human medical community must be accompanied by a corresponding effort among veterinarians, farmers and others in the food animal and companion animal industries.The overuse of potent antibiotics for non-bacterial disease is a major reason for resistance.4 Physicians are pressured by patients to prescribe them in spite of evidence of non-bacterial signs and symptoms. Patients start treatment and then stop prematurely when their symptoms subside, allowing the less susceptible bacteria to survive, thus producing a strain resistant to traditionally effective treatments. Furthermore, overuse and misuse can allow bacteria of different species and even different genus to transfer resistance genes. For instance, research shows antibiotic resistance, once acquired, disseminates throughout Enterococci, via horizontal transfer of mobile genetic elements, and confers vancomycin resistance from Enterococci to MRSA.5
In the case of eye disease, optometrists often reach for the latest and greatest antibiotic for non-sight-threatening conditions when an older antibiotic would have better efficacy. This overuse of current-generation antibiotics for minor infections exposes common pathogens to new antibiotics and can hasten the development of resistant strains. Even worse, many clinicians prescribe antibiotics for prophylaxis when in many cases no real need exists. This not only leads to an increase in resistance among common ocular flora, but also toxicity from the use of an unnecessary therapeutic agent.
Bugs and Drugs
The World Health Organization, the Food and Drug Administration and the Centers for Disease Control and Prevention all monitor antibiotic resistance trends among bacterial pathogens. The organisms that produce ocular disease are rarely targets of these investigations. It wasn’t until 2008 that results were available from the first multicenter, nationwide antibiotic resistance surveillance program specific to ocular pathogens.6 The first of these, the Ocular Tracking Resistance in the US Today (Ocular TRUST) study, annually evaluates, in vitro, the susceptibility of three bacterial species—Staphylococcus aureus, S. pneumonia and H. influenza—to several antibiotics: ciprofloxacin, gatifloxacin, levofloxacin, moxifloxacin, penicillin, azithromycin, tobramycin, trimethoprim and polymyxin B in national samples of ocular isolates.6The study reported antibiotic resistance among ocular isolates of the test organisms collected between 2005 and 2006 from 35 institutions across the United States. The study found that 16.8% of S. aureus isolates were methicillin resistant (MR), with many isolates concurrently resistant to other antibiotic classes. A significant proportion of pneumococcal isolates had intermediate resistance to penicillin (18.3%), azithromycin (22.4%) and trimethoprim (22.4%), whereas no notable resistance was reported for H. influenza isolates. Results of the second and third years of the Ocular TRUST study (TRUST 2 and TRUST 3) showed methicillin resistance among S. aureus isolates increased to nearly 50% in 2008 and methicillin resistance among coagulase-negative Staphylococci (CoNS) was as high as 62.0%. Results for S. pneumonia and H. influenza appeared unchanged.6
The Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) study was initiated in 2009 to survey antibiotic resistance among S. aureus, CoNS, S. pneumonia, H. influenza and Pseudomonas aeruginosa isolates from ocular infections.7 As in the Ocular TRUST study, ARMOR was a multicenter, nationwide prospective surveillance study and provided resistance data to extend the information gleaned from Ocular TRUST.7 It presents antibiotic resistance profiles and trends for more than 3,000 ocular isolates collected during the first five years of the ARMOR study. The isolates, which included 1,169 S. aureus, 992 CoNS, 330 S. pneumoniae, 357 H. influenza and 389 P. aeruginosa, were collected from 72 eye care centers, community hospitals and university hospitals across 36 states.7 Gram-positive isolates such as Staphylococcus and Streptococcus species came primarily from adults and, as would be expected, H. influenza was primarily isolated from children younger than 10 years.7 H. influenza represents the most common ocular pathogen in the pediatric population. In ARMOR, pseudomonal isolates were most common in patients between 10 to 29 years old.7
 |
| Fig. 3. This patient’s chlamydial conjunctivitis will likely require topical therapy. Photo: Gary E. Oliver, OD |
Of the 1,169 S. aureus isolates, 465 (39.8%) and 743 (63.6%) were resistant to ciprofloxacin and azithromycin, respectively. In addition, 227 isolates (19.4%) were resistant to tobramycin. All S. aureus isolates were susceptible to vancomycin, and only a small proportion were resistant to trimethoprim (4.7%) and chloramphenicol (0.4%).7
Regarding Streptococcus species, only azithromycin has a significant rate of failure. The resistance was found to be 34%, much higher than all other drugs tested.7 The gram-negative H. influenza species in children was found to be susceptible to all test drugs.7 More information that can be derived from the Ocular TRUST and ARMOR studies can be found in Table 2.
Of greatest importance: the rise of resistant among the most common adult pathogens—coagulase negative and positive Staphylococcus species—the high failure rate of fluoroquinolones and the efficacy of older drugs like trimethoprim and tobramycin.7
Table 2. Resistance in MRSA and Pseudomonas7 | |
| S. aureus – MRSA | % Resisitant |
| Ofloxacin | 76% |
| Ciprofloxacin | 76% |
| Levofloxacin | 72% |
| Gatifloxacin | 68% |
| Moxifloxacin | 57% |
| Besifloxacin | NA |
| Azithromycin | 93% |
| Chloramphenicol | 0.7% |
| Tobramycin | 41% |
| Trimethoprim | 7% |
| Vancomycin | 0% |
| Pseudomonas | |
| Ofloxacin | 6% |
| Ciprofloxacin | 5% |
| Levofloxacin | 4% |
| Gatifloxacin | NA |
| Moxifloxacin | NA |
| Besifloxacin | NA |
| Tobramycin | 3% |
| Polymyxin B | 3% |
Disease Factors
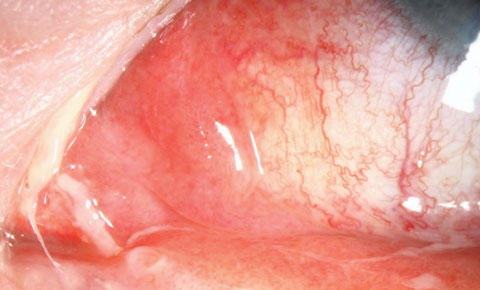
Selecting an antibiotic therapy is highly dependent on the risk to the patient. We don’t want to undertreat—or overtreat, as low-risk infections can often be treated empirically with a host of topical broad-spectrum agents. However, in the case of higher risk infections, which include bacterial keratitis, postoperative refractive surgery infections, periocular infections and prophylaxis prior to procedures, specific protocols are required to ensure we meet the standard of care. Preseptal cellulitis, dacryoadenitis, dacryocystitis, internal hordeola and chlamydial infections may require both systemic and topical therapy (Figures 1, 2 and 3).A significant risk factor for ocular infectious disease is contact lens use. Despite the development of new materials and lens care systems, ODs must deal with the unpredictable human factor. Poor hygiene, dirty, dusty work environments, smoking, overwear of contact lenses and failure to replace lenses according to recommended schedules are all risk factors for developing potentially serious corneal infections.
Most non-sight-threatening ocular disease is treated empirically. If the condition is serious enough to threaten visual function, it’s imperative to determine the identity and susceptibility of the organism. For instance, sight-threatening keratitis may require the compounding of specific antibiotics for their appropriate therapy (Figure 4).3 These include the compounded forms of vancomycin, amikacin and azithromycin (Table 3).
Topical vs. Systemic Therapy
Ocular infections can be unique in that, in many cases, topical therapy is preferred over systemic. Topical therapy allows for a highly concentrated dose of medication to be applied directly to the site of infection. The benefits are obvious: minimal systemic absorption, reduction in systemic toxicity and direct delivery of high drug concentration to avascular tissues (i.e., the cornea). Systemic medications are necessary when infections involve deeper, vascularized structures such as the lids, periorbital area and lacrimal apparatus.The choice of drug depends on the pathogen and the patient’s history, which includes allergies, preexisting medical conditions and the patient’s current medications. Another major consideration for using systemic medications is pregnancy. Doctors must select an effective medication that does not pose a risk to the fetus. The FDA’s classification system can help clinicians review the risk of medications in pregnant patients (Table 4). High-risk diseases warrant high-risk treatments and discussions with the patient, family doctor and OB/GYN. Otherwise, clinicians should avoid high-risk treatments in low-benefit situations.
The Drugs
A host of new and old agents are currently available to help us combat infectious disease. Many have become obsolete while others that had fallen out of favor have been resurrected as useful therapies.Aminoglycosides. These drugs are bactericidal and their efficacy is concentration dependent, both desirable characteristics of a topical anti-infective.8 They inhibit bacterial ribosomes, the workhorses of cellular protein synthesis.
Tobramycin is the most commonly prescribed aminoglycoside, a category which also includes two topical drugs—neomycin and gentamicin—and the newest aminoglycoside, amikacin (no ophthalmic form is currently available).3 Amikacin is generally compounded to treat tobramycin-resistant Pseudomonas. Topical neomycin is highly sensitizing, and gentamicin has significant corneal toxicity.9 Tobramycin is mostly popular for its anti-pseudomonal activity.3 The ophthalmic form is available as a 0.3% solution (3mg/cc), but can be compounded in a concentration as high as 13.5mg/cc for use in suspected gram-negative corneal infections.
Its use is also limited by its corneal toxicity—it’s now been replaced by topical fluoroquinolone agents, which are effective against most gram-negative bacteria, including Pseudomonas, without the toxicity issues and the need to be compounded. Overall, we don’t see many indications for tobramycin today. Trimethoprim, however, shows high efficacy against MRSA and is less toxic for management of non-sight threatening conjunctivitis.6 Vancomycin is the drug of choice for sight-threatening resistant Staphylococcus species.
Trimethoprim is a synthetic anti-infective agent. Like the sulfonamides, it is a folic acid inhibitor, but mediates its effects slightly differently; it’s also safe for sulfonamide-sensitive patients.10
Its activity is limited to gram-positive bacteria.3 Therefore, it is usually combined with a drug that has gram-negative activity like polymyxin B. This combination has broad-spectrum activity and low toxicity, and it is a good option to empirically treat bacterial conjunctivitis in all age groups. It is bacteriostatic, not bactericidal, and is a time-dependent antibiotic. This means the concentration in tissues must remain above the organism’s MIC90 level (the minimum concentration needed to inhibit growth of 90% of the isolates present) for a specific period of time for it to be effective. Trimethoprim has recently made a comeback for managing non-sight-threatening MRSA conjunctivitis.7 It exhibits low toxicity, high efficacy against gram-positive bacteria—specifically MRSA—and is inexpensive.
Polymyxin B is a topical peptide antibiotic and a bactericidal cell wall inhibitor effective against Pseudomonas, Escherichia coli, Enterobacter and Klebsiella.3 The more effective fluoroquinolone agents have supplanted its use in cases of serious sight-threatening gram-negative infection.
Bacitracin, once a popular bactericidal topical antibiotic, is rarely used today for several reasons. First, it is a narrow-spectrum drug with efficacy only for gram-positive organisms such as Staphylococcus and Streptococcus species.3 Second, it frequently produces contact dermatitis reactions.3 Finally, it is only available as an ointment, which most patients dislike due to greasiness and blurred vision.3
Erythromycin and azithromycin. These are both available in topical and oral dosage forms. These drugs represent the macrolide protein synthesis class of antibiotics. Topical use of erythromycin was once quite common. It had good gram-positive activity and was effective against Chlamydia trachomatis. It also has very low corneal toxicity. Unfortunately, it is only available in ointment form for topical use. And, its ineffectiveness against H. influenza and propensity to irritate the GI tract has caused it to fall out of favor.
Erythromycin is bacteriostatic, with limited efficacy against pediatric Haemophilus.11 Oral erythromycin interferes with the hepatic metabolism of drugs metabolized by the cytochrome P-450 system, whereas azithromycin does not.12 Erythromycin is notorious for producing GI irritation, while once-daily azithromycin rarely does.12 Because of its marked ability to quickly treat chlamydial infections (a single dose of 1,000mg in adults), long half-life, lack of hepatic drug interactions and efficacy against Haemophilus, azithromycin has become one of the most popular oral antibiotics for treating ocular disease.11 Furthermore, it can be used safely in both pregnant patients and those allergic to penicillins.3
Note that azithromycin is not effective in treating MRSA infections because of a greater than 90% resistance seen in the ARMOR study.13 It is commonly used to treat marginal blepharitis due to reported anti-inflammatory properties.3
 |
| Fig. 4. Keratitis, seen here, requires aggressive therapy to stave off vision loss. Photo: Christine Sindt, OD |
The topical formulations of these agents revolutionized the topical management of ocular infectious disease. They possessed the ideal characteristics of an anti-infective agent. They are bactericidal, concentration-dependent, rarely produce sensitization or toxicity and are broad-spectrum agents. Of course, due to these favorable characteristics, they were—and are still—widely overprescribed. Furthermore, the majority of the fluoroquinolones used are for prophylaxis in agricultural animals.14 Broad exposures of the drugs to the biosphere and the food chain has resulted in a significant reduction in their efficacy. This is particularly problematic because resistance has primarily developed in gram-positive Staph. species.7
In an effort to combat resistance, new generations of FQs have been developed.
The first drugs approved to treat bacterial keratitis were ciprofloxacin and ofloxacin. Improvements in third-generation FQs included a purified version of ofloxacin, a 50/50 mixture of left- and right-handed stereoisomers, though only the left-handed isomer was biologically active. By producing a purified left-handed isomer, levofloxacin, they greatly lowered the required MIC90.
The fourth-generation fluoroquinolones adds a methoxy functional group to the fluoroquinolone structure, making it more effective against resistant gram-positive organisms, and decreasing resistance. The fourth-generation drugs include moxifloxacin 0.5% and gatifloxacin 0.3% and 0.5%. Both drugs are available in oral formulations, but they are rarely used systemically to treat eye disease.
Besifloxacin represents an improved version of the FQ drug class. In addition to fluorine attached to the quinolone ring, an atom of chlorine is attached as well. Furthermore, this drug is not used orally or in agriculture. Current studies of efficacy are limited, but have demonstrated reduced resistance in FQ-resistant bacteria.15
Vancomycin. This is a bactericidal cell wall inhibitor with specificity for gram-positive bacteria.12 It is compounded as a 25mg to 50mg/mL topical form or an intraocular injection to treat MRSA endophthalmitis and is the principal drug used to treat serious ocular MRSA infections.13,16 The ARMOR study showed it was 100% effective against ocular MRSA isolates; however, vancomycin-resistant strains of MRSA have surfaced.
Chloramphenicol. This is an extremely potent inhibitor of bacterial ribosomal protein synthesis. It is a bacteriostatic, broad-spectrum antibiotic.3 Its spectrum of activity is impressive. It is effective against gram-positive and gram-negative bacteria, anerobic and aerobic organisms, tick-borne rikettsiae and MRSA.3 It is highly lipid soluble and therefore has excellent tissue penetration when used topically, and can pass through the blood-brain barrier and blood-eye barrier when it is used systemically.3
Chloramphenicol is widely used to topically treat eye disease in many parts of the world due to its high efficacy and low cost.17 Its use in the United States for eye disease is limited due to its systemic toxicity in both systemic and topical dosage forms. It has the potential to produce fatal aplastic anemia at a rate of one case in 24,000 of treatment courses.18
Amoxicillin-clavulanate (Augmentin). This is a combination of the broad-spectrum aminopenicillin amoxicillin and clavulanate, a non-anti-infective compound that binds to the enzyme beta-lactamase. Clavulanate’s ability to inhibit beta-lactamase improves efficacy against organisms traditionally resistant to penicillin therapy. This includes beta-lactamase producing Staphylcoccus, Streptococcus and Haemophilus bacteria.3 It is not effective against MRSA strains. The drug is a bactericidal, cell wall-inhibiting antibiotic. It is well tolerated and safe in pregnancy.19 The major issue with it and other penicillins is the significant number of individuals allergic to this class of therapeutic agent.19
Cephalosporins. This group of oral agents is very similar to the aminopenicillins. They inhibit cell walls and are bactericidal.3 Compared to the penicillins, they exhibit improved resistance to beta lactamase, but are not as effective as clavulanate-protected amoxicillin.19 Given an approximate 3% incidence of cross-sensitivity with penicillins, cephalosporin use in penicillin-sensitive patients should be limited.3
Over time, researchers have developed several generations of cephalosporins. The most commonly prescribed first-generation drug, cephalexin, is primarily effective against gram-bacteria in adults.20 When treating children younger than 10 years of age who tend to colonize Haemophilus, clinicians should use a second-generation cephalosporin.21
Table 3. Antibiotic Selection for Sight-threatening Pathogens | ||
| Vancomycin | 50mg/cc | MRSA |
| Gatifloxacin or Moxifloxacin | 0.5mg/cc | Pseudomonas |
| Tobramycin | 13.5mg/cc | Pseudomonas |
| Amikacin | 50mg/cc | Pseudomonas |
| Solomon R, Donnenfeld ED, Holland EJ, et al. Microbial keratitis trends following refractive surgery: results of the ASCRS infectious keratitis survey and comparisons with prior ASCRS surveys of infectious keratitis following keratorefractive procedures. J Cataract Refract Surg. 2011;37(7):1343-50. | ||
Disinfectants
These compounds have the ability to kill bacteria, virus, fungi and protozoa. Their use is limited primarily by their toxicity.22Povidone-iodine, a 21-iodine-based disinfectant, has become quite popular for disinfection of the eye and adnexa prior to surgical procedures.22 It has also been used in its 5% ophthalmic dosage form to treat adenoviral conjunctivitis.23 A povidone-based product is currently in clinical trials for the treatment of both viral and bacterial eye disease. The drug contains a combination of povidone-iodine 0.4% and dexamethasone 0.1%.24
Non-ophthalmic Topicals
Mupirocin, a combination of pseudomonal acids sold as Bactroban (GlaxoSmithKline), is available as a topical ointment and cream. It is profoundly effective against gram-positive bacteria, most importantly MRSA. It is the topical drug of choice for impetigo and MRSA skin infections and is administered nasally in MRSA carriers to reduce their contagion potential.25 It is not approved for ophthalmic use, but certainly could be used off-label on lids and periocular infected skin.Table 4. FDA Pregnancy Category of Risk of Selected Antibiotics3 | |
| Tobramycin (topical) | B |
| Fluoroquinolones (topical) | C |
| Trimethoprim/polymyxin B (topical) | C |
| Cephalosporins | B |
| Azithromycin | B |
| Amoxicillin-Clavulanate | B |
| Chloramphenicol (topical) | C |
In short: In an age of flourishing bacterial resistance to antibiotics, know the patient, know the drug and know the disease before treating any infection.
Dr. Onofrey is a clinical professor at the University of Houston College of Optometry and is an author of The Ocular Therapeutics Handbook.
|
1. Bertino JS. Impact of antibiotic resistance in the management of ocular infections: the role of current and future antibiotics. Clin Ophthalmol. 2009;3:507-21. 2. Chang VS, Dhaliwal DK, Raju l, Kowalski RP. Antibiotic resistance in the treatment of Staphylococcus aureus keratitis: a 20 year revue. Cornea. 2015;34(6):698-703. 3. Onofrey BE, Skorin, L, Holdeman NR. The Ocular Therapeutics Handbook. 3rd ed. Lippincott Williams and Wilkins; 2011. 4. Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance: The need for global solutions. Lance Infec. Dis. 2013;13(12):1057-98. 5. Palmer KL, Kos VN, Gilmore MS. Horizontal gene transfer and the genomics of enterococcal antibiotic resistance. Curr Opin Microbiol. 2010;113(5):632-9. 6. Asbell PA, Colby KA, Deng S, et al. Ocular trust: Nationwide antibiotics susceptibility patterns in ocular isolates. Amer J Ophthalmol. 2008;145(6):951-8. 7. Asbell PA, Sanfilippo CM, Pillar CM, et al. Antibiotic resi stance among ocular pathogens in the United States (ARMOR). JAMA Ophthalmol. 2015;133(12):1445-54. 8. Poole K. Aminoglycoside resistance in Pseudomonas aeruginosa. Antibiotics Agents Chemother. 2005;49(2):479-87. 9. Kaye D. Current for old antibacterial agents: Polymyxins, rifampin and aminoglycosides. Infect Dis Clin North Am. 2004;18(3):669. 10. Chambers HF, Deck DH. Sulfonamides, trimethoprim and Quinolones. In: Katzung, BG, Master SB, Trevor AJ. Basic and Clinical Pharmacology. 11th ed. 2009; Lange. 11. Gilbert DN, Moellering RC, Eliopoulos GM, et al, eds. The Sanford guide to antibiotics therapy. 46th ed. Antimicrobial Therapy; 2016. 12. Chambers HF, Deck DH. Tetracyclines, macrolides, clindamycin, chloramphenicol, streptogramins and oxazolidinones. In: Katzung, BG, Master SB, Trevor AJ, eds. Basic and Clinicical Pharmacology. 11th ed. McGraw-Hill Medical; 2009. 13. Asbell PA, Sahm DF, Shaw M, et al. Increasing prevalence of methicillin resistance in serious ocular infections caused by Staph. aureus in the United States 2000-2005. J Cat Refract Surg. 2008;34(5):814-18. 14. Gustafson RH, Bowen RE. Antibiotic use in animal agriculture. J App Microbiol. 1997;83(5):531-41. 15. Sanders ME, Norcross EN, Moore QC. Efficacy of besifloxacin in a rabbit model of methicillin resistant Staphylococcus aureus keratitis. Cornea. 2009;10(28):1055-60. 16. Manav K, Pathenga A, Mathai A, et al. Vancomycin resistant gram positive bacterial endophthalmitis: epidemiology, treatment options and outcomes. J Ophthalmol Inflam Infec. 2013;3:46. 17. Walker S, Diaper CJ, Bowman R, et al. Lack of evidence of systemic toxicity following topical chloramphenicol use. Eye. 1998;12( Pt 5):875-9. 18. McWhae JA, CChang J, Lipton JH. Drug induced fatal aplastic anemia following cataract surgery. Can J Ophthalmol. 1992;27(6):313-5. 19. Nahum GG, Uhl K, Kennedy DL. Antibiotic use in pregnancy and lactation: What is and is not known about teratogenic and toxic risks. Obs Gynec. May 2006;107(5):1120-38. 20. Dancer SJ. The problem with cephalosporins. J Antibio chemother. 2001;48(4):463-78. 21. Rathore M. Pediatric Haemophilus infection. Emedicine.medscape.com/article/964317-overview. MedScape. Updated April 4, 2016. Accessed March 30, 2017. 22. Isenberg SJ, Apt L. The ocular application of povidone iodine. Comm Eye Health J. 2003;16(46):30-1. 23. Abel R, Abel AD. Use of povidone-iodine in the treatment of presumptive adenoviral conjunctivitis. Ann Ophthalmol Glaucom. 1998;30(6):341-3. 24. Pinto RD, Lira RP, Abe RY, et al. Dexamethasone/Povidone Eye Drops versus Artificial Tears for Treatment of Presumed Viral Conjunctivitis: A Randomized Clinical Trial. Curr Eye Res. 2015 Sep;40(9):870-7. 25. Lewis l. Impetigo treatment and management. MedScape. Emedicine.medscape.com/article/965254-treatment. Updated May 4, 2016. Accessed March 30, 2017. |

