As a progressive eye disease, glaucoma is on every optometrist’s radar, especially primary open-angle glaucoma (POAG), the most common form.1-10 Research estimates it will affect around 80 million people worldwide by the year 2020.6,7,11 The main therapeutic goal for patients diagnosed with POAG is slowing disease progression and the rate of visual field loss—accomplished by reduction of intraocular pressure (IOP) with medical therapy.1,3,6,8,10-13
These days, however, medical therapy isn’t as simple as prescribing eye drops and sending patients on their way. Clinicians mainly prescribe from one of four classes of IOP-lowering medications: beta blockers (B-blockers), carbonic anhydrase inhibitors (CAIs), prostaglandin analogs (PGAs) and alpha 2-adrenergic agonists.2,7,9,14
Each class can cause local and systemic adverse reactions, and clinicians must take all of them into consideration when choosing the right therapy for each patient (Table 1).4,8,9,15,16 And when patients don’t see the IOP lowering effects they need with one class of drug, clinicians can add a second or even a third drug, which further complicates the treatment plan. This article discusses the many IOP-lowering medication options and factors that can influence treatment choices.
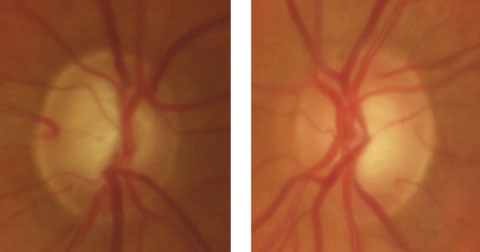 |
| This 69-year-old white male has no family history of glaucoma. His initial IOP was 42mm Hg in the right eye and 36mm Hg in the left. With brimonidine BID, dorzolamide BID and latanoprost QHS, his IOP is now 15mm Hg in both eyes. Click image to enlarge. |
Physiology and Mechanism of Action
Aqueous humor, produced at the ciliary processes, is regulated by neuro inputs from both the sympathetic and parasympathetic systems and by vascular contractile-dilation in the ciliary body. Aqueous is produced by diffusion, ultrafiltration and active secretion, involving the active transport of Na+, Cl- and HCO3-. Aqueous production occurs in the posterior chamber and passes out of the anterior chamber through the trabecular meshwork. Most resistance to aqueous outflow is located in the extracellular matrix (ECM) of the trabecular meshwork. Glycosaminoglycans within the ECM influence the hydration of the trabecular meshwork, and the parasympathetic cholinergic innervation of the iris and the ciliary muscle all influence aqueous humor outflow.
Aqueous outflow is also assisted by an unconventional route through the uveal meshwork and the ciliary muscle called the uveoscleral pathway. Prostaglandin F2α within the ciliary muscle decreases the flow resistance of its interstitial space, thereby increasing aqueous outflow through the uveoscleral pathway. B-blockers and alpha agonists reduce aqueous production by their effects on the B2 adrenergic and presynaptic α2 receptors, respectively. Alpha agonists also can increase trabecular meshwork outflow. CAIs inhibit the activities of carbonic anhydrase responsible for HCO3- secretion, thus reducing the production and active secretion of aqueous humor from the ciliary body. Prostaglandin analogs increase uveoscleral outflow by activating prostaglandin F2α receptors, leading to ECM remodeling in the ciliary muscle, in turn reducing hydraulic resistance and increasing uveoscleral outflow.9,16
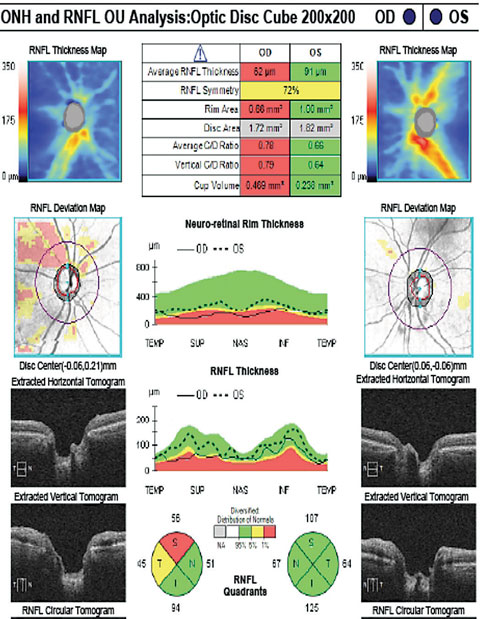 |
| The patient’s RNFL scan shows superior and temporal rim thinning in the right eye. Click image to enlarge. |
Where to Begin
PGAs are often first-line treatment for IOP reduction in POAG and ocular hypertension (a condition with elevated IOP but no detectable glaucoma damage) and are recommended by both the American Academy of Ophthalmology Preferred Practice Patterns and the UK-based National Institute for Health and Care Excellence guidelines.1,9,12,17-19
For some, PGA monotherapy is enough to achieve and maintain adequate IOP control. For many, however, more than one medication is required to achieve desirable IOP reduction, and clinicians must consider adding a B-blocker, a CAI and/or an alpha agonist to an existing PGA.3,13,20
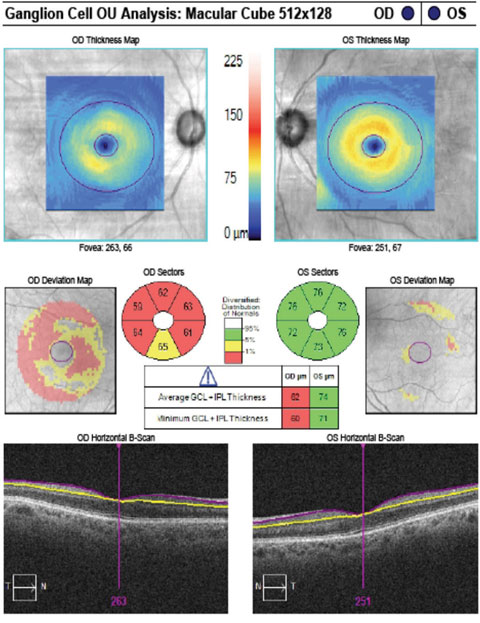 |
| The patient’s GCA correlates with rim thinning in the right eye and a normal left eye. Click image to enlarge. |
Two is Better than One
Research has yet to outline which adjunctive agents are most effective in achieving IOP control. Some monotherapy clinical trials suggest that B-blockers and alpha agonists are more effective than CAIs in IOP control. However, a systemic review and meta-analysis revealed similar mean diurnal IOP-lowering efficacy when a B-blocker, an alpha agonist or a CAI agent was combined with a PGA.21 One study found adding a CAI to a PGA lowered nocturnal IOP more than with either a B-blocker or alpha agonist.21
In lieu of better clinical data, clinicians must take factors such as efficacy, frequency of dosing schedule, ocular side effects and tolerability into consideration when prescribing an additional medication.8,21 Research suggests incidence of eye pain and burning sensation is higher with alpha agonists and CAIs compared with B-blockers, which can affect patient compliance.21 Another major advantage of using a B-blocker as adjunctive therapy is its FDA-approved once-daily dosing of timolol 0.5% gel forming solution, although generally FDA-approved dosing for B-blockers is BID.
Aqueous production drops at night, which may explain why B-blockers are ineffective in nocturnal IOP reduction. Most research indicates timolol has the greatest IOP-lowering efficacy in the morning, while PGAs are most effective in the evening. Clinicians may prescribe a concomitant therapy of timolol 0.5% gel daily in the morning and PGA at night for optimal IOP reduction—limiting the dosing frequency to only twice a day. Also, one study found timolol causes less severe ocular side effects with higher tolerability, which may increase patient compliance with medication adherence.
CAIs are good alternative agents to add to PGAs for patients who are contraindicated to B-blockers or if the IOP-lowering effects are not satisfactory. Topical CAI agents are FDA-approved for TID dosing, but are often used BID in clinical practice, especially as adjunctive therapy. Another option for therapy with PGAs is an alpha agonist. Like CAIs, alpha agonists are also FDA-approved for TID dosing, but are often used BID. Some studies have suggested the alpha agonist brimonidine in particular provides a neuroprotective role, which may slow down the progressive loss of retinal ganglion cells in glaucoma.3,5,9,13,21
Contraindications |
| All IOP-lowering medications possess some degree of local and systemic side effect. Some, however, are contraindicated for patients with specific systemic conditions. Patients with asthma, chronic obstructive pulmonary disease, bradycardia, heart block, congestive heart failure or those taking an oral beta blocker should not be treated with a topical B-blocker.4,8,9 PGAs should be avoided by those who are pregnant or have an ophthalmic inflammatory condition. Alpha agonists are contraindicated in neonates, children younger than two and those who are taking monoamine oxidase inhibitors.15,16 |
Third Time’s the Charm
Unfortunately, some patients still do not achieve adequate IOP control with two adjunctive medications. In these cases, treatment becomes more complicated when a third topical hypotensive agent is added.
Some of the challenges with multiple drug therapy include an increase in dosing frequency, risk of drug washout, ocular side effects and exposure to preservatives, the latter causing an increase in ocular surface disease and discomfort. These factors can potentially interfere with medication adherence and decrease overall efficacy.14,22,23 When patients are placed on multiple concomitant hypotensive agents, clinicians should consider fixed-combination medications as an alternative to traditional concomitant therapy.10,19
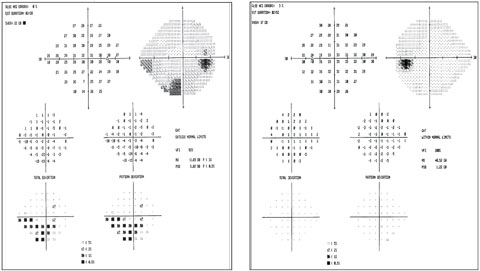 |
| Visual fields show the inferior arcuate in the right eye that correlates with RNFL and GCA. The fields are clean in the left eye. Click image to enlarge. |
Combinations, Simplified
Most fixed-combination medications contain a B-blocker with a CAI, an alpha agonist or a PGA—only one combines a CAI with an alpha agonist. In the United States, only three fixed-combinations are FDA-approved: Cosopt (dorzolamide/timolol, Akorn) BID, Combigan (brimonidine/timolol, Allergan) BID and Simbrinza (brinzolamide/brimonidine, Alcon) TID.13,24
One of the main advantages of fixed-combination is dosing frequency. Concomitant treatment involving a B-blocker (BID) and a CAI (BID or TID) or an alpha agonist (BID or TID) requires patients instill two separate medications in the morning and in the evening for a total of four drops per day, five minutes between drops—which can be troublesome and time consuming. Fixed-combination therapy can reduce dosing frequency to two or three drops (when already on a PGA) per day. Less daily dosing simplifies a patient’s treatment plan, avoids medication washout, decreases preservative exposure and, in many cases, decreases ocular effects without affecting IOP-lowering efficacy.3,10,13,20,25
Here is a closer look at the combined mechanisms of action for the available fixed-combination medications (Table 2):
B-blocker/CAIs work synergistically to reduce overall aqueous production.3
B-blocker/alpha agonists also work synergistically as an aqueous suppressant. Alpha agonists can also enhance outflow through the uveoscleral pathway—perhaps further reducing IOP.3
CAI/alpha agonists decrease aqueous production and increase uveoscleral outflow. It is often a good alternative treatment for those who cannot take B-blockers or wish to avoid PGAs due to ocular effects of hyperemia, eyelash growth, iris or periorbital hyperpigmentation, especially with monocular treatments.13
B-blockers/PGA lower IOP by decreasing aqueous production and increasing outflow.3,10,20
Table 1. Glaucoma Drug Options4,7,8,12,15,16 | ||||
| Class | Names | Mechanism of Action: Reduce IOP | Ocular Effects | Systemic Effects |
| Beta-blocker | •Timolol •Betagan (levobunolol, Allergan) •Ocupress (carteolol, Novartis) •Betoptic (betaxolol, Alcon) •Optipranolol (metipranolol, •Valeant Pharmaceuticals) | •Decrease aqueous production | •Conjunctival allergy •Hyperemia •Corneal epithelial disorders •Reduced corneal sensitivity •Blurry vision | •Decrease blood pressure/pulse •Bradycardia •Worsen asthma/COPD •Depression •Impotence •Lethargy |
| Carbonic anhydrase inhibitor | •Trusopt (dorzolamide, Merck) •Azopt (brinzolamide, Alcon) •Diamox (acetazolamide oral capsule, Teva Pharmaceuticals) •Neptazane (methazolamide oral capsule, Perrigo) | •Decrease aqueous production | •Same as B-blockers •Ocular irritation •Foreign body sensation | Topical use: •Bitter taste •Fatigue •Diuresis •Gastrointestinal upset Oral use: •Nausea •Unpleasant taste •Dysesthesia of fingers/lips •Anorexia •Metabolic acidosis |
| Prostaglandin analog | •Xalatan (latanoprost, Pfizer) •Travatan (travoprost, Alcon) •Rescula (unoprostone isopropyl, Sucampo) •Zioptan (tafluprost, Akorn) --------------------------- •Lumigan (bimatoprost, Allergan) | •Increase uveoscleral outflow ----------------------- •Increase uveoscleral and trabecular meshwork outflow | •Same as B-blockers •Eyelash growth •Iris/eyelid pigment •Deepening of upper eyelid sulcus •Recurrence of herpes •Macular edema post cataract surgery | •Rarely, upper respiratory infection •Rarely, myalgia |
| Alpha 2-adrenergic agonist | •Alphagan (brimonidine, Allergan) •Iopidine (apraclonidine, Alcon) | •Decrease aqueous production •Increase uveoscleral outflow | •Allergic conjunctivitis •Hyperemia •Mydriasis •Dry eye | •Affects blood pressure/pulse •Drowsiness •Dizziness •Dry mouth •Dysarthria |
| Parasympath-omimetic/ cholinergic- agonist | •Pilocar (pilocarpine, FDC Limited) | •Increase trabecular meshwork outflow | •Miosis •Visual field constriction •Night vision loss •Myopia •Red eye •Brow ache •Retinal detachment •Cataract | Direct-acting: •Rare systemic reactions Indirect-acting: •Sweating •Tearing •Nausea/vomiting •Diarrhea •Bradycardia •Stomach ache |
Treatments on the Horizon
Because glaucoma is the second leading cause of blindness worldwide, researchers are continually on the hunt for better therapeutic options.1,3,7,8,12,14 These new meds are designed to simplify treatment with a once-daily dosing schedule:
Vyzulta (latanoprostene bunod 0.024%, Bausch + Lomb) is a compound of latanoprost and a nitric oxide (NO) donor that reduces IOP by increasing aqueous outflow through both the trabecular meshwork/Schlemm’s canal and uveoscleral pathways. In phase III trials, once-daily use of this drug performed better than both twice-daily timolol and once-daily latanoprost. Mild punctate keratitis and ocular hyperemia are the most common ocular side effects.2,4,9,14,26-28
Rhopressa (netarsudil 0.02%, Aerie Pharmaceuticals) is both a RHO-associated protein kinase inhibitor and norepinephrine transporter inhibitor. It has two mechanisms of action aimed at IOP reduction: increasing trabecular meshwork outflow and decreasing aqueous production. Rho-kinase inhibitors destabilize filamentous actin, leading to more empty space in trabecular meshwork and improving outflow. Norepinephrine transporter inhibitors result in reduced aqueous production. It has a once-daily dosing schedule, and the most common ocular side effect is mild hyperemia.2,4,9,11,14,28 A phase III clinical trial was completed in 2016.
Roclatan (Aerie Pharmaceuticals), currently in phase III clinical trials, is a combination of netarsudil 0.02% and latanoprost 0.005%. It is administered once daily to act on both the trabecular meshwork and the uveoscleral outflow pathways to reduce fluid production.9,14,24,28
Trabodenoson (Inotek Pharmaceuticals) is an adenosine analog that targets A1 receptors, resulting in the removal of proteins from the trabecular meshwork, lowering outflow resistance and IOP.9,14,29 Researchers are working to combine trabodenoson with latanoprost and brimonidine for glaucoma treatment.2,4,9,14,28 In January 2017, Inotek announced the results of phase III clinical trials, which did not achieve superiority to placebo at all 12 time points.30
Table 2. Fixed-combination Medications3,4,8,13,24 | ||
| Effects on aqueous humor (AH) | Market availability | |
| Cosopt (dorzolamide/timolol, Akorn) | Decrease AH/decrease AH | USA/other countries |
| Combigan (brimonidine/timolol, Allergan) | Decrease AH, increase outflow/decrease AH | USA/other countries |
| Simbrinza (brinzolamide/brimonidine, Alcon) | Decrease AH/decrease AH, increase outflow | USA/other countries |
| Brinzolamide/timolol | Decrease AH/decrease AH | Other countries |
| DuoTrav (travoprost/timolol, Alcon) | Increase outflow/decrease AH | Canada/other countries |
| Latanoprost/timolol | Increase outflow/decrease AH | China/other countries |
| Bimatoprost/timolol | Increase outflow (uveoscleral and TM)/decrease AH | China/countries worldwide |
| PGA/alpha agonist/B-blocker | Increase outflow/decrease AH | Mexico |
Because the only way to manage glaucoma is to reduce the primary risk factor—elevated IOP—clinicians must be prepared to prescribe any number of IOP-lowering drugs, as monotherapy or in combination. PGAs have emerged as the gold standard initial monotherapy treatment for POAG; but when monotherapy cannot achieve desirable IOP reduction, additional medications, whether through concomitant therapy or fixed-combination medications, can help adequately control a patient’s IOP. In the future, newer treatment modalities may lower IOP and slow glaucoma progression with even better dosing regimens. n
Dr. Yee is a staff optometrist in the Salisbury VA Health Care System, Salisbury, NC.
| 1. Fung DS, Whitson JT. An evidence-based review of unoprostone isopropyl ophthalmic solution 0.15% for glaucoma: place in therapy. Clinical Ophthalmology. 2014;8:543-54. 2. Rocha-Sousa A, Rodrigues-Araujo J, Gouveia P, et al. New therapeutic targets for intraocular pressure lowering. ISRN ophthalmology. 2013;2013:261386. 3. Radcliffe NM. The impact of timolol maleate on the ocular tolerability of fixed-combination glaucoma therapies. Clinical Ophthalmology. 2014;8:2541-9. 4. MK. Present and new treatment strategies in the management of flaucoma. The Open Ophthalmology Journal. 2015;9:89-100. 5. Doozandeh A, Yazdani S. Neuroprotection in glaucoma. J Ophthal Vision Res. 2016;11(2):209-20. 6. Knight OJ, Lawrence SD. Sustained drug delivery in glaucoma. Curr Opin Ophthalmol. 2014;25(2):112-7. 7. Wojcik-Gryciuk A, Skup M, Waleszczyk WJ. Glaucoma -state of the art and perspectives on treatment. Restorative Neurology and Neuroscience. 2015;34(1):107-23. 8. Inoue K. Managing adverse effects of glaucoma medications. Clinical Ophthalmology. 2014;8:903-913. 9. Bucolo C, Platania CB, Reibaldi M, et al. Controversies in glaucoma: current medical treatment and drug development. Current Pharmaceutical Design. 2015;21(32):4673-81. 10. Fang Y, Ling Z, Sun X. Fixed-combination treatments for intraocular hypertension in Chinese patients - focus on bimatoprost-timolol. Drug Design, Development and Therapy. 2015;9:2617-25. 11. Ting NS, Li Yim JF, Ng JY. Different strategies and cost-effectiveness in the treatment of primary open angle glaucoma. ClinicoEconomics and Outcomes Research. 2014;6:523-30. 12. Li T, Lindsley K, Rouse B, et al. Comparative effectiveness of first-line medications for primary open-angle glaucoma: a systematic review and network meta-analysis. Ophthalmology. 2016;123(1):129-40. 13. Sharma S, Trikha S, Perera SA, Aung T. Clinical effectiveness of brinzolamide 1%-brimonidine 0.2% fixed combination for primary open-angle glaucoma and ocular hypertension. Clinical Ophthalmology. 2015;9:2201-7. 14. Schehlein EM, Novack GD, Robin AL. New classes of glaucoma medications. Curr Opinion Ophthalmoly. November 2016. [Epub ahead of print]. 15. Rhee DJ. Glaucoma: color atlas and synopsis of clinical ophthalmology. Wills Eye Hospital. 2003. 16. Bartlett JD. Clinical ocular pharmacology. Fourth ed. Boston: Butterworth-Heinemann; 2001. 17. Peeters A, Schouten JS, Severens JL, et al. Latanoprost versus timolol as first choice therapy in patients with ocular hypertension. A cost-effectiveness analysis. Acta ophthalmologica. 2012;90(2):146-54. 18. Daka Q, Trkulja V. Efficacy and tolerability of mono-compound topical treatments for reduction of intraocular pressure in patients with primary open angle glaucoma or ocular hypertension: an overview of reviews. Croatian Medical Journal. 2014;55(5):468-80. 19. Singh K, Lee BL, Wilson MR. A panel assessment of glaucoma management: modification of existing RAND-like methodology for consensus in ophthalmology. Part II: Results and interpretation. Am J Ophthalmol. 2008;145(3):575-81. 20. Barnebey HS, Robin AL. Adherence to fixed-combination versus unfixed Travoprost 0.004%/timolol 0.5% for glaucoma or ocular hypertension: a randomized trial. Am J Ophthalmol. December 2016. [Epub ahead of print]. 21. Tanna AP, Lin AB. Medical therapy for glaucoma: what to add after a prostaglandin analogs? Curr Opin Ophthalmol. 2015;26(2):116-20. 22. Hollo G, Topouzis F, Fechtner RD. Fixed-combination intraocular pressure-lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expert Opinion on Pharmacotherapy. 2014;15(12):1737-47. 23. Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS). Invest Ophthalmol Vis Sci. 2007;48(11):5052-7. 24. Fechtner RD, Khouri AS. Fixed combination: a mainstay of glaucoma management today and tomorrow. Glaucoma Today. 2016;14(6):33-6. 25. Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308-16. 26. Araie M, Sforzolini BS, Vittitow J, Weinreb RN. Evaluation of the effect of latanoprostene bunod ophthalmic solution, 0.024% in lowering intraocular pressure over 24 h in healthy Japanese subjects. Advances in Therapy. 2015;32(11):1128-39. 27. Cavet ME, Vollmer TR, Harrington KL, et al. Regulation of endothelin-1-induced trabecular meshwork cell contractility by latanoprostene bunod. Invest Ophthalmol Vis Sci. 2015;56(6):4108-16. 28. Glaucoma Research Foundation. New medical therapies for glaucoma. 2017. www.glaucoma.org/treatment/new-medical-therapies-for-glaucoma.php. Accessed February 17, 2017. 29. Myers JS, Sall KN, DuBiner H, et al. A dose-escalation study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of 2 and 4 weeks of twice-daily ocular trabodenoson in adults with ocular hypertension or primary open-angle glaucoma. J Ocular Pharmacol Therapeutics. 2016;32(8):555-62. 30. Inotek announces top-line results for MATrX-1, first phase 3 trial of trabodenoson for galucoma. BusinessWire. January 3, 2017. www.businesswire.com/news/home/20170103005518/en/Inotek-Announces-Top-line-Results-MATrX-1-Phase-3. Accessed March 22, 2017. |

