Systemic Therapy vs. Implant for Uveitis
Long-term follow up reveals systemic therapy may be better for chronic uveitis patients.
By Rebecca Hepp, Managing Editor
Patients with chronic uveitis may consider systemic therapy with corticosteroids and immunosuppressants in lieu of a long-term corticosteroid intraocular implant, according to new research. The study, funded by the National Eye Institute, found that, after seven years of treatment, patients on systemic therapy had stable visual acuity, while those with the implant saw a decline of roughly six letters.
The study looked at 255 patients with uveitis randomly assigned either a fluocinolone intraocular implant, or systemic therapy consisting of prednisone and immunosuppressants such as methotrexate or mycophenolate mofetil. While visual acuity remained about the same in the two groups through two years, researchers noted reactivations of uveitis after roughly five years in the implant-treated eyes. This coincided with a decline in visual acuity, which the researchers speculate may be due to increased damage in the retina and choroid.
In addition to the long-term changes in treatment efficacy, patients with the implant were more likely to experience negative ocular effects, such as cataracts, elevated intraocular pressure and glaucoma. Patients receiving systemic therapy, although they had an increased risk of needing treatment with antibiotics, did not have large increases in the risk of adverse effects common with systemic corticosteroids such as high blood pressure or diabetes.
“This study now clearly states that systemic oral therapy is just as good, and in fact better, than a steroid implant,” says Nathan Lighthizer, OD, an associate professor at Oklahoma College of Optometry. “This may save the patient another visit or a referral to a specialist since optometrists in some states can prescribe oral steroids.”
But Dr. Lighthizer still recommends ODs consult with an ophthalmologist or uveitis specialist for some refractory cases, which may lead to a team-based care approach for some patients. “It is good to know that these specialists now may be more inclined to treat with systemic therapy rather than surgery, and we may need to participate in the comanagement of these patients and potentially follow them long-term.”
“Anytime a surgical implant into the eye can be avoided, it’s potentially a good thing, especially when oral therapy in this study proved to preserve more vision, have fewer long-term side effects and was more cost-effective,” Dr. Lighthizer adds. “That is a win all around.”
Bill Leaves Essential Vision Benefits Uncertain
The American Health Care Act (AHCA) that passed the House on May 4 is facing criticism from patient groups such as the American Medical Association and the American Association of Retired Persons for potentially rolling back popular health care benefits.1,2 For optometry specifically, care for the youngest patients may soon be in flux.
A Legislative Win in GeorgiaOptometrists in Georgia can now perform certain injections, thanks to the passage of SB 153, which was signed into law by Governor Nathan Deal on May 9, 2017. ODs ready to take on the scope of practice expansion must first attend a 30-hour injectables training program approved by the board and be under the direct supervision of a board-certified ophthalmologist, according to the bill. The bill also includes a provision allowing optometrists to treat ocular pain with non-narcotic oral analgesics, hydrocodone administered orally and Schedule III or Schedule IV oral analgesics. Georgia General Assembly. 017-2018 Regular Session - SB 153. www.legis.ga.gov/Legislation/en-US/display/20172018/SB/153. Accessed June 2, 2017. |
As it currently stands, the bill provides an option for states to independently decide whether to maintain, suspend or make changes to the essential benefits previously protected by the Affordable Care Act (ACA)—including pediatric vision care.3 The change is an attempt to “encourage fair health insurance premiums,” according to the bill.3
When the ACA was passed in 2010, pediatric vision was included as one of the 10 essential benefits that must be covered by all providers.4 That coverage included a yearly eye exam with a materials benefit for every patient younger than 19.5
The legislation passed the House after an amendment written by Rep. Tom MacArthur (NJ) and Rep. Mark Meadows (NC) resolved intraparty disagreements.3 The bill still faces Senate scrutiny and is now in limbo after a Congressional Budget Office report suggested 24 million could lose coverage.5
1. Gurman A. AMA statement on cbo score of american health care act. American Medical Association. May 24, 2017. www.ama-assn.org/ama-statement-cbo-score-american-health-care-act. Accessed May 30, 2017. |
Get Ready for DEWS II
Attendees at this year’s Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, held in Baltimore from May 6-11, were the first to get a glimpse of The Tear Film & Ocular Surface Society’s (TFOS) forthcoming Dry Eye Workshop II (DEWS II) recommendations. The full report sets out to update the definition, classification and diagnosis of dry eye, as well as evaluate its impact, address management and therapy and develop recommendations for clinical trials to better assess treatment options.1
DEWS II took two years to complete and involved 150 experts from around the world, who used an evidence-based approach to increase our understanding of dry eye disease, said organizer David Sullivan, PhD, in a press release.1
While the report’s updated definition for dry eye—which adds a focus on homeostasis—is a significant change today, its call for better research on treatment outcomes is a welcome addition for the future, according to Lyndon Jones, OD, chair of the DEWS II Management and Therapy Subcommittee and a professor at the Centre for Contact Lens Research, School of Optometry and Vision Science, University of Waterloo.
“What really shocked us was what little high-level evidence there is to support many of the things we do or we prescribe on a day-to-day basis,” said Dr. Jones at the ARVO briefing. “And even when we have decided whether the patient has aqueous deficient or evaporative dry eye, how we then tailor that management and therapy to specifically treat those two just simply is missing. We need more evidence. We knew [dry eye treatment] was complex before, and what we really need is a huge number of new studies.” Noting that a decade has passed since the first DEWS, Dr. Jones joked that “the good thing is, we’ll be busy for the next decade.”
After two years of work, DEWS II will be published July 1 by The Ocular Surface and will be available at www.tearfilm.org, according to the ARVO presentation.
| 1. Tear Film & Ocular Surface Society. www.tearfilm.org/dettnews-tfos_dews_ii_report_announced/101_16/eng. Accessed May 30, 2017. |
Cancer Drug Combats Thyroid Eye Disease
A drug targeting the insulin-like growth factor 1 receptor may help reduce symptoms associated with thyroid eye disease, according to a new report.1
Teprotumumab, originally tested as a cancer treatment, was investigated in a multicenter study for its ability to reduce the severity of thyroid-associated ophthalmopathy.1 Researchers randomly assigned 87 patients, diagnosed nine months or less prior to the onset of symptoms, to receive placebo or intravenous teprotumumab once every three weeks, over a 24-week period, or for eight injections total.1
The study defined a response as a reduction of two points on a seven-point clinical activity score and a reduction of at least two millimeters in proptosis at the end of week 24.1
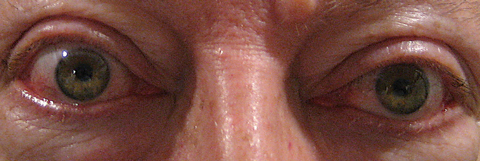 |
| This patient exhibits proptosis from thyroid orbitopathy. Click image to enlarge. Photo: Michael Trottini, OD |
In patients who received teprotumumab, 69% achieved a response vs. 20% of those who received placebo at week 24, and 43% of patients in the teprotumumab group achieved relief of their symptoms within six weeks.1 The drug was also well tolerated, and the hyperglycemia some patients experienced was well-controlled after adjusting the dosage.1
However, only patients recently diagnosed with active, moderate to severe disease were enrolled in the study, so further investigation is needed to asses teprotumumab’s ability to provide relief in patients with stable or milder forms of the disease. Also, orbital imaging was not performed to determine the specific tissues affected by teprotumumab therapy. A one-year follow up is currently underway to determine the drug’s long-term therapeutic effect.
“The results of the study are quite impressive—there is a marked clinical difference in response in the group receiving the active drug compared with placebo, and the onset of improvement was rapid,” says Tammy Than, OD, a professor at the University of Alabama at Birmingham School of Optometry. “FDA was impressed as well, as last year it granted this drug ‘breakthrough therapy’ designation.”
However, a few questions remain, and Dr. Than hopes further research will help uncover whether regression of clinical improvement occurs once the infusions stop.
The researchers say teprotumumab may also help patients with other autoimmune conditions with ocular manifestations.1
“Since other applications may exist for teprotumumab in rheumatoid arthritis and other autoimmune diseases, this drug may offer an avenue of high impact for managing numerous diseases that manifest with debilitating ocular sequelae,” Dr. Than concludes.
| 1. Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Eng J Med. 2017;376(18):1748. |
In the NewsBausch + Lomb recently announced updated results from the ARMOR surveillance study, including preliminary 2016 data on antibiotic resistance levels and an eight-year trend analysis of antibiotic resistance among staphylococcal isolates. They found non-susceptibility to fluoroquinolones more than doubled from 2015, and methicillin resistance is decreasing amongS. aureus, but not among coagulase-negative staphylococci. While resistance is decreasing, resistance to several commonly used antibiotics is still a challenge, according to Penny Asbell, MD, lead author. Bausch + Lomb Reports Updated Results of the Antibiotic Resistance Monitoring in Ocular MicRoorganisms (ARMOR) Study. May 10, 2017.www.bausch.com/our-company/recent-news/id/2374/5102017-Wednesday. Accessed May 16, 2017. The Optometric Glaucoma Society recently established the Optometric Glaucoma Foundation (OGF) to support glaucoma education for the optometric profession, including students, residents, educators and practitioners. The 501(c)(3) not-for-profit organization is designed to promote excellence in the care of glaucoma patients, support research and help optometrists become involved in research. The OGF is led by Murray Fingeret, OD, president; Leo Semes, OD, vice president; John McSoley, OD, secretary; and Austin Lifferth, OD, treasurer. Researchers have found that roughly 15% of Ebola survivors in Sierra Leone who had previously reported ocular symptoms have a retinal scar that seems unique to the disease. Researchers now speculate the virus enters the eye through the optic nerve to reach the retina, similarly to West Nile Virus. Steptoe PJ, Scott JT, Baxter JM, et al. Novel retinal lesion in Ebola survivors, Sierra Leone, 2016. Emerging Infectious Diseases. 2017;23(7). [Epub ahead of print]. |

