 |
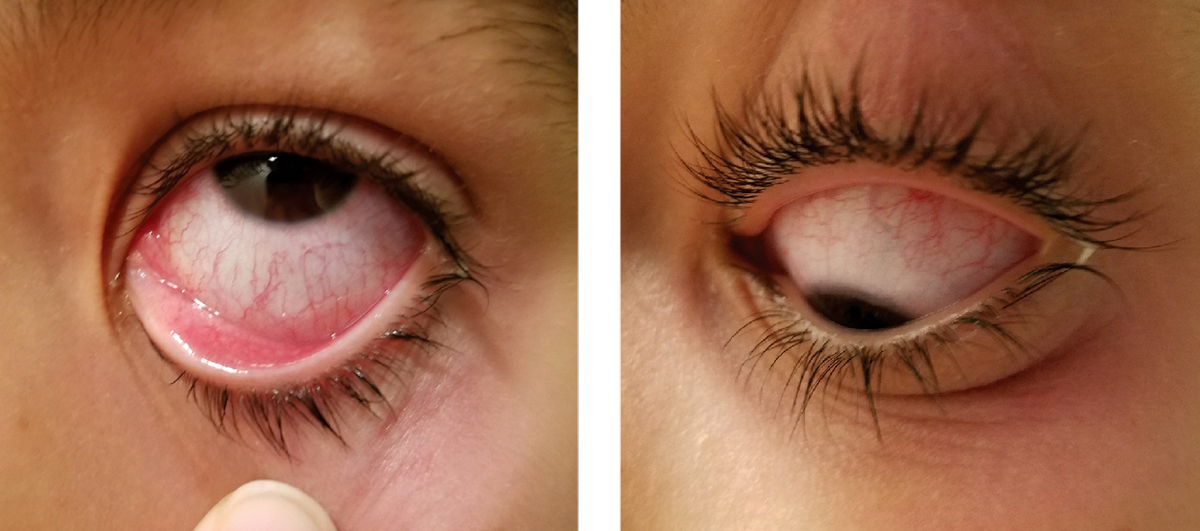
| Many schools require students with “pink eye” to be prescribed an antibiotic before returning and that alone may argue for the intervention. However, a literature review found only marginal clinical benefit from it. Photo: Alexandra Espejo, OD. Click image to enlarge. |
As generous prescribing of broad-spectrum antibiotics can lead to resistance, a group of researchers wanted to specify if their use was warranted in cases of conjunctivitis. Summarizing the key findings from a Cochrane Review for American Journal of Ophthalmology, the group analyzed the benefits and safety of antibiotic therapy compared with a placebo in cases of acute bacterial conjunctivitis.
Included were 21 randomized controlled trials that compared topical antibiotic use with a placebo in a total of 8,805 patients. Most of these trials (71%) examined using fluroquinolone (FQ) drops; three tested macrolides either alone or with combination steroids and three others compared non-FQ antibiotics.
Intention-to-treat (ITT) estimates suggested antibiotics may increase clinical recovery by 26% at the end of therapy, increased treatment completion rates and reduced persistent clinical infection after one course of treatment when compared with placebo. When looking only at laboratory-confirmed cases of bacterial conjunctivitis, antibiotics were associated with 53% higher likelihood of microbiological cure compared with placebo, as well as with better treatment adherence.
Non-FQ therapies increased only microbiological, and not clinical, cure efficacy. However, non-FQs were shown likely to increase treatment-associated ocular complications, including eye pain, discomfort and allergic reactions, while FQs did not, but the certainty of evidence for this was low due to potential risk of bias from study design and inconsistent outcome measurement and reporting.
Giving more detail, the study authors specify that these findings may be more applicable to acute bacterial conjunctivitis in the older pediatric and adult population that for neonatal bacterial conjunctivitis, since neonatal cases require systemic antibiotics, as well as the common cause being different in the non-neonatal pediatric vs. adult population (Haemophilus influenzae vs. Staphylococcus aureus, respectively).
The moderate certainty of evidence found in the benefits of topical antibiotic use may be called into question, though, due to self-limited nature and relatively low morbidity of most cases. The authors point out that while an individual pays for cost of a doctor’s visit and antibiotic prescription and rarely may suffer adverse events, the costs and adverse events are multiplied on a societal level.
Even further, topical FQs lead to resistance, which is of concern, since fourth-generation fluoroquinolones are what’s used for vision-threatening bacterial keratitis. Although ocular concentration of topical antibiotics differ from systemic concentrations of antibiotics, tropical resistance may carry over systemically.
Interestingly, intention-to-treat results showed that 55.5% of placebo participants spontaneously resolved clinically by days four to nine, compared with the rate of 68.2% in the antibiotic treatment group. The authors pose that this may argue against the requirement that many schools maintain for a child with conjunctivitis to have been prescribed for conjunctivitis before returning to school.
Although this offers a good general understanding, the authors add that “future research is required to assess the clinical and microbiological efficacy among different antibiotic classes, bacterial species or treatment durations of the same antibiotic in head-to-head trials.”
Liu SH, Chen YY, Nurmatov U, van Schayck OCP, Kuo IC. Antibiotics vs. placebo for acute bacterial conjunctivitis: findings from a Cochrane Systematic Review. Cochrane Database Syst Rev. 2023. [Epub ahead of print]. |

