Ongoing technological advances, new drug formulations and increased scope of practice have all collided to allow optometrists the opportunity to manage difficult cases that were once previously referred to our ophthalmology counterparts. These five disease presentations—vitreomacular adhesion (VMA) and traction (VMT), post-op cystoid macular edema (CME), neurotrophic keratitis (NK), traumatic brain injury (TBI) and glaucoma/ocular hypertension (OHTN)—are commonly evaluated in the optometric setting and can be managed successfully, in most cases, without referral. Case examples help illustrate how optometrists can independently treat and manage each condition.
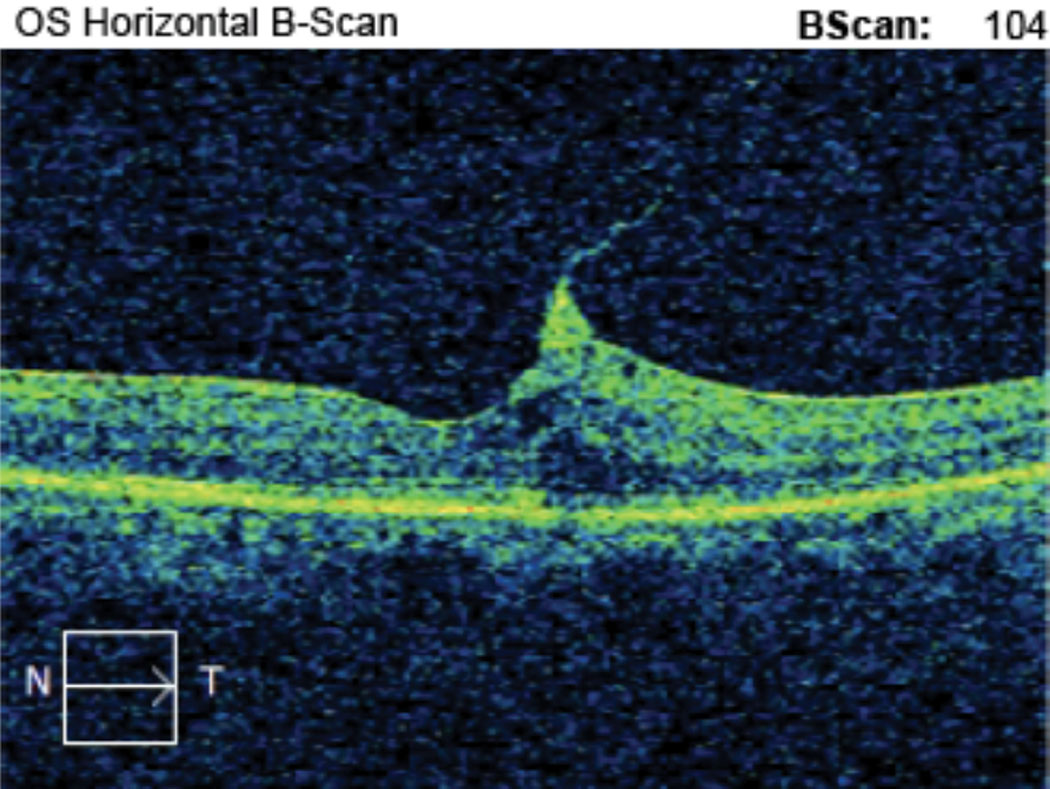 |
| Case 1. This is the horizontal OCT B-scan of the left eye of an 85-year-old black male patient with VMT who is being monitored. BCVA is 20/50- OS. He reports metamorphopsia on the Amsler grid but denies scotoma. We discussed monitoring vs. surgical consultation, and the patient prefers to self-monitor with Amsler grid. He is being followed every three months with serial macular OCT and dilated fundus exam. Click image to enlarge. |
VMA and VMT
In adults, the average volume of the vitreous body is 4mL.1 The vitreous is contained in the posterior chamber of the eye in a cortex that abuts the posterior lens capsule, ciliary body, optic nerve and internal limiting membrane (ILM) of the retina. As we age, gel liquefaction occurs within the vitreous body, causing a weakening of the vitreoretinal adhesions posteriorly.1,2 This biochemical change manifests clinically as a posterior vitreous detachment (PVD) and can be ongoing for decades. A PVD occurs when there is vitreous separation from the macula and optic nerve.1
Pathophysiology. VMA is described as a perifoveal vitreous detachment in the presence of persistent foveal attachment observed on ocular coherence tomography (OCT).1 VMA is a variant of normal given the physiological presence of vitreoretinal adhesion. The observance of VMA with OCT represents a single stage of impending vitreous separation from the retina without underlying abnormalities (i.e., intraretinal cysts).1 The International Vitreomacular Traction Study Group has subclassified VMA into two types: focal and broad.1 Focal VMA occurs when there is less than or equal to 1,500µm of vitreous adhesion overlying the ILM, while broad VMA occurs when the adhesion is greater than 1,500µm.1 Patients with VMA typically do not report visual symptoms and have the potential for the condition to spontaneously resolve without retinal sequelae.
VMT is the attachment of the vitreous cortex to the ILM in the area of the macula, resulting in anatomic abnormality of the area. Like VMA, there are two types: focal and broad.1 The condition is focal when the size of the vitreous-ILM adhesion is less than or equal to 1,500µm and broad when the adhesion is greater than 1,500µm.1
VMT occurs when there is an imbalance between vitreous liquefaction and the separation of the vitreous cortex from the retina.3,4 The result of this mismatch is an anomalous PVD—an attachment of the vitreous to the ILM in the macular region. The area of the anomalous PVD has subsequent tractional deformation given the continued vitreous attachment anteriorly.1
VMT is more likely to affect females than males and has the highest incidence in the sixth decade of life.5 The estimated prevalence of VMT is 22.5 cases per 100,000 persons.6 Unlike VMA, patients with VMT often have visual complaints such as decreased visual acuity (VA), metamorphopsia, blurred vision, micropsia and photopsia.2,7
 |
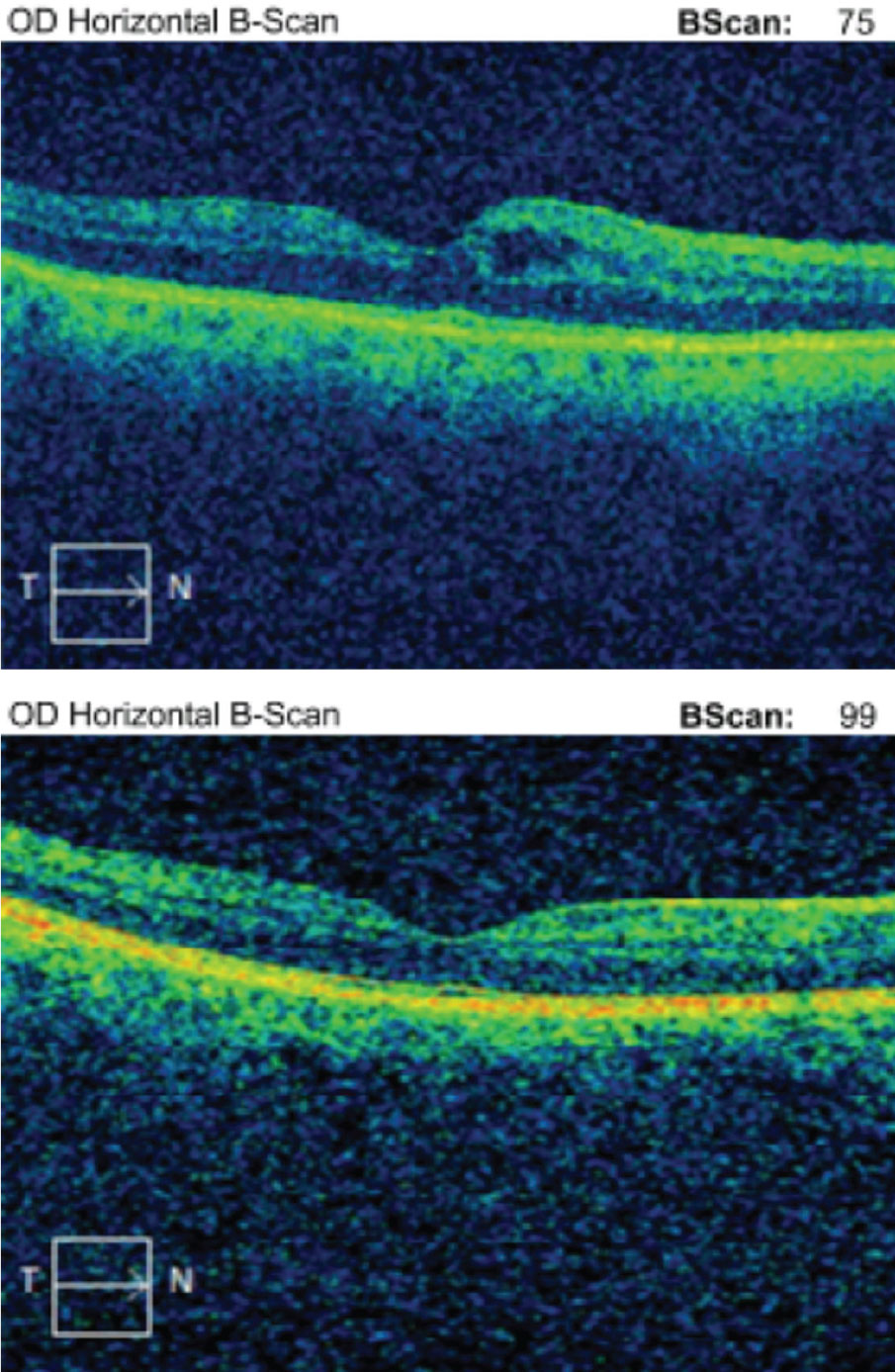
| Case 2. Here is a macular OCT of a 64-year-old black female with focal VMA in the right eye. BCVA is 20/20 OD. The patient is asymptomatic for metamorphopsia or scotoma and is being monitored yearly. Click image to enlarge. |
Management. Most, if not all, cases of VMA can be monitored conservatively. Given that VMA may be a transient aging change and the vitreous may likely separate from the ILM without issue, referral to a retina specialist is not warranted. Patients with VMA have no underlying changes to the foveal umbo so VA is preserved, except in the setting of other macular and retinal conditions. Conservatively, patients can be given a home Amsler grid to test monocularly on a frequent basis. Patients should be instructed to call the office immediately should they notice changes such as metamorphopsia and scotoma. Follow-up for VMA would be six months to a year with a full dilated fundus examination and macular OCT.
Management of VMT mirrors that of VMA in cases where the patient is asymptomatic for metamorphopsia and scotoma and VA is 20/60 or better in the affected eye.
Interestingly, a systematic review and meta-analysis of the safety and efficacy of pars plana vitrectomy (PPV) for VMT found a mean improvement in VA to 20/53 postoperatively.8
Additionally, a retrospective case series that reviewed the prognostic factors of postoperative intraretinal cystoid spaces (ICS) after primary PPV for VMT found that preoperative ICS was a major risk factor for persistent ICS post-op.2 Thus, patients with VMT with or without intraretinal cysts with a VA of 20/60 or better can be appropriately managed in the optometric practice. A macular OCT should be done with a dilated fundus exam at three- to six-month intervals to assess for changes in VMT, VA and symptoms.
Prior to the patient leaving, perform an in-office Amsler grid to demonstrate how to use the grid and to note any pre-existing metamorphopsia or scotoma. Should either be present, make note of this and educate the patient that they are to return should they notice any changes from the current findings (Cases 1 and 2).
 |
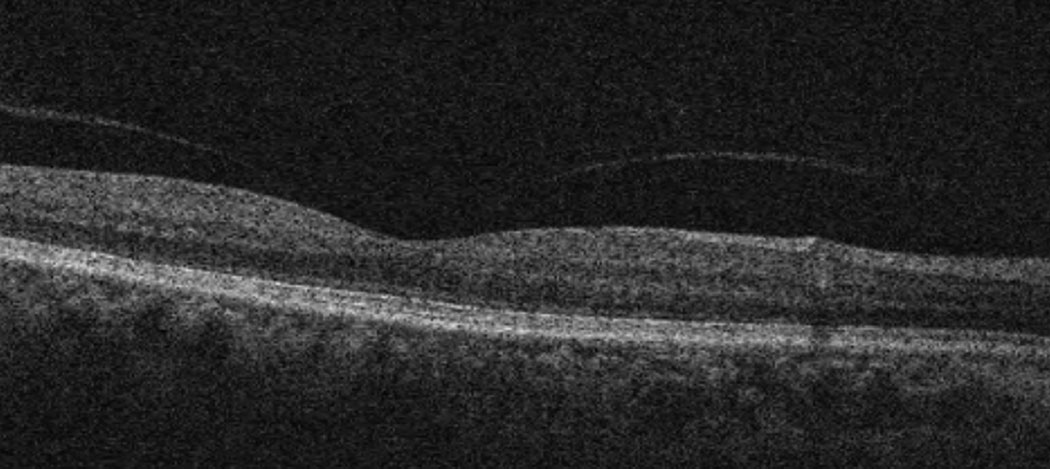
| Case 3. Above is the initial macular OCT scan of a 64-year-old black female who developed IGS nine weeks post-op in the right eye. BCVA was 20/40. Below is her macular OCT scan after 11 weeks of difluprednate 0.05% and ketorolac 0.5% therapy in the right eye. Her BCVA resolved to 20/20. Click image to enlarge. |
Post-op CME
In 1953, Irvine-Gass syndrome (IGS) was first reported for CME following cataract surgery, followed by the first fluorescein angiography (FA) diagnosis in 1969.9,10 IGS typically occurs within 12 weeks with a peak incidence between weeks four and six after cataract surgery.11
The diagnosis is made with clinical exam of the fundus, FA, macular OCT or a combination of all three. With macular OCT imaging, intra-retinal spaces are observed in the area of and around the foveal umbo. Patients with IGS will report decreased VA and contrast sensitivity and metamorphopsia in the affected eye(s). Advancements in cataract removal have dramatically reduced the prevalence of post-op complications, and the prevalence of IGS in those without risk factors is 1% to 2%.12
Pathophysiology. Surgically induced inflammation is the root etiology of IGS. During surgery, activation of phospholipase A2 (PLA2) propagates the inflammatory pathway by up-regulating arachidonic acid (AA) production. AA production results in eicosanoid (i.e., prostaglandin) and leukotriene synthesis via the cyclooxygenase (COX) and lipoxygenase (LOX) pathways, respectively.13 These inflammatory mediators, produced in the anterior chamber during cataract surgery, have the potential to migrate posteriorly to the retina.14 When this occurs, the inflammatory mediators cause vasodilation, vascular permeability and disruption of the blood-retinal barrier.15
Management. IGS can effectively be treated with a combination of topical corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs). Corticosteroids inhibit PLA2, down-regulating the production of AA and subsequent substrates for both COX and LOX enzymes.16 Corticosteroids also have the ability to inhibit vasodilation, decrease vascular permeability and stabilize intracellular and extracellular membranes.17 NSAIDs work downstream of the inflammatory cascade by inhibiting the COX enzymes, thereby decreasing eicosanoid formation.
Often, patients with IGS will already have these drugs in their arsenal for both pre- and post-operative use. Each drug’s formulation will dictate the specific dosing of both the corticosteroid and NSAID. For generic prednisolone and ketorolac, QID dosing of both medications is warranted.
Clinicians can schedule follow-up with VA, intraocular pressure (IOP), fundus examination and macular OCT in two- to four-week intervals until complete resolution is noted. If IGS is persistent past 12 weeks of topical treatment, consider increasing the topical prednisolone to Q1H while awake.
In one clinical trial, there was a statistically significant difference in best-corrected visual acuity (BCVA) and macular thickness 12 weeks after changing the dosing frequency of prednisolone to Q1H while awake.18 It is important to slowly taper both the corticosteroid and NSAID to prevent the recurrence of intraocular inflammation (Case 3).
 |
| Case 4. A 78-year-old white male with stage two NK in the left eye was successfully treated with Oxervate over a 56-day treatment course. The patient had a history of herpes zoster with persistent epithelial defects for which he underwent a partial lateral tarrsorhaphy OS. Post-Oxervate therapy, there was full epithelialization, and the tarrsorphaphy was removed 90 days later due to continued corneal health and patient interest. Special thanks to Patrick McMannamon, OD, for the case. Photo: Patrick McMannamon, OD. Click image to enlarge. |
Neurotrophic Keratitis
Corneal sensation is important to maintain homeostasis of the ocular surface by mediating the production of trophic factors that support and maintain the corneal epithelium and corneal nerves.19 NK is an uncommon corneal disease in which the sensory-mediated reflexes are reduced. The estimated prevalence of NK is 1.6 to 4.2 cases per 10,000 persons.19,20
Currently, the disease is classified into three stages. Stage one manifests with punctate epithelial keratitis, epithelial hyperplasia, stromal scarring and corneal neovascularization.21,22 Stage two is a persistent epithelial defect.21 The most severe, stage three, is corneal stromal ulceration that may result in corneal perforation or melting.21,22 NK can result from past ocular infections (herpes being the most common), chemical corneal burns, ocular surgeries, topical medications, cranial nerve V palsy and select systemic diseases.22,23
Diagnosis of NK is confirmed quantitatively or qualitatively with in-office corneal sensitivity testing.
Pathophysiology. Corneal epithelial turnover and transparency is due to the highly innervated network of corneal nerves.24 The human cornea is the most innervated structure in the body—40 times more innervated than tooth pulp and 400 times more innervated than human skin.25 Corneal stem cell proliferation and differentiation is mediated by the secretion of neurotrophins released by the trigeminal nerve.24 In cases of NK, decreased amounts of nerve growth factor (NGF), substance P, calcitonin gene-related peptide, neuropeptide Y and acetylcholine are significantly reduced.24 The reduction of neurotrophins results in both morphological and metabolic epithelial disruptions leading to ocular surface defects and non-healing corneal wounds.20 Additionally, the ocular surface suffers further desiccative stress from poor tear re-establishment due to reduced blink rates.
Management. Treatment and management of NK is dependent on the stage with more aggressive and surgical interventions usually reserved for stage three. Therapy for stage one includes use of artificial tears, most often preservative-free if the drop is used frequently throughout the day to decrease preservative-induced ocular surface toxicity.
Stage two NK requires the use of corneal or scleral therapeutic contact lenses, amniotic membrane transplantation, topical autologous serum drops and topical recombinant human NGF.20,24,26 One study found both amniotic membrane treatment and conventional treatment (tarrsorhaphy and a bandage contact lens) were both effective options for refractory neurotrophic corneal ulcers.27
Stage three NK often requires surgical intervention to prevent corneal perforation. In 2018, Oxervate (cenegermin-bkbj 0.002%, Dompé), a topical human NGF, was FDA approved for the treatment of NK. In a multicenter, randomized, vehicle-controlled pivotal trial, researchers found that Oxervate had statistically higher rates of corneal healing compared with the vehicle when dosed six times a day over a 56-day period.26
The complete healing of the corneal epithelium is paramount in the management of NK to prevent risk of ulceration and perforation. Management and follow up will depend on the stage of the condition and the modality of treatment instituted. For more severe cases of NK, daily follow up may be warranted—at least initially—until complete epithelial healing has occurred (Case 4).
Case 5. Treat TBI With LensesA 45-year-old Hispanic male, a social worker, presented to the TBI clinic for evaluation of eye strain, eye pain, sensation of increased eye pressure and headaches after hitting his head on a wooden table while trying to plug in his computer. He reported limited ability in reading for a sustained period of time, although his job requires extended computer and near activities. Exam findings were remarkable for CI and accommodative dysfunction in both eyes. The patient was successfully treated with separate distance and near glasses and reported increased comfort and duration with near tasks. This case demonstrates a Tier 2 management of mTBI with successful visual comfort with the use of lenses. Special thanks to Siva Meiyeppen, OD, for the case. |
Traumatic Brain Injury
With the increased awareness of the visual sequela after a TBI, optometrists play a key role in the inter-professional management team. Approximately 2.8 million people in the United States sustained a TBI in 2013, most commonly as a result of a fall, being struck by or against an object or a motor vehicle accident.28 Additionally, with US troops arriving back from the Middle East, an increased prevalence of TBI can be expected from returning active duty service members.
Optometrists are well positioned to manage the resulting visual impairments for patients with mild TBI (mTBI), a closed head injury with loss of consciousness <30 minutes, a Glasgow Coma Score of 13 or greater, post-traumatic amnesia <24 hours, altered level of awareness <24 hours and normal neural imaging studies.29 Visual symptoms of mTBI are exacerbated at near with complaints of visual acuity fluctuations, headaches and photophobia with near work.30 This is not surprising given binocular vision diagnoses of accommodative dysfunction, convergence insufficiency (CI) and saccadic dysfunction have been reported most frequently in the literature.30,31
Management. Researchers suggest a four-tiered conceptual model for the management of mTBI to address the visual, psychological and neurological sequelae:
- Tier 1 is the basic optometric visual examination where the patient’s refractive, binocular visual system and ocular health are evaluated.32
- Tier 2 is the detection of oculomotor-based vision problems via version, vergence and accommodative evaluation.32 The abnormalities found dictate whether lenses for distance and near, vision therapy and/or prism are indicated.
- Tier 3 is non-oculomotor based vision problems.32 In this tier, the patient’s spatial localization, motion sensitivity, photosensitivity, vestibular function, visual fields and visual information processing are evaluated.32 Treatments for the non-oculomotor vision problems may include prism, yoked prism, tints, binasal occlusion, vision/vestibular therapy and visual information processing and perceptual therapy.32
- Tier 4 addresses non-vision based problems.32 The patient is screened for depression, fatigue, cognitive impairment and behavioral, postural, attention and neurological problems.32 Based on pertinent concomitant issues, the patient may need evaluation and treatment by psychiatry, psychology, counseling and neurology.32
The overall success of the patient may require multiple assessments of the different aspects of the visual system, vision information processing system and inter-collaborative care with other professionals (Case 5).32
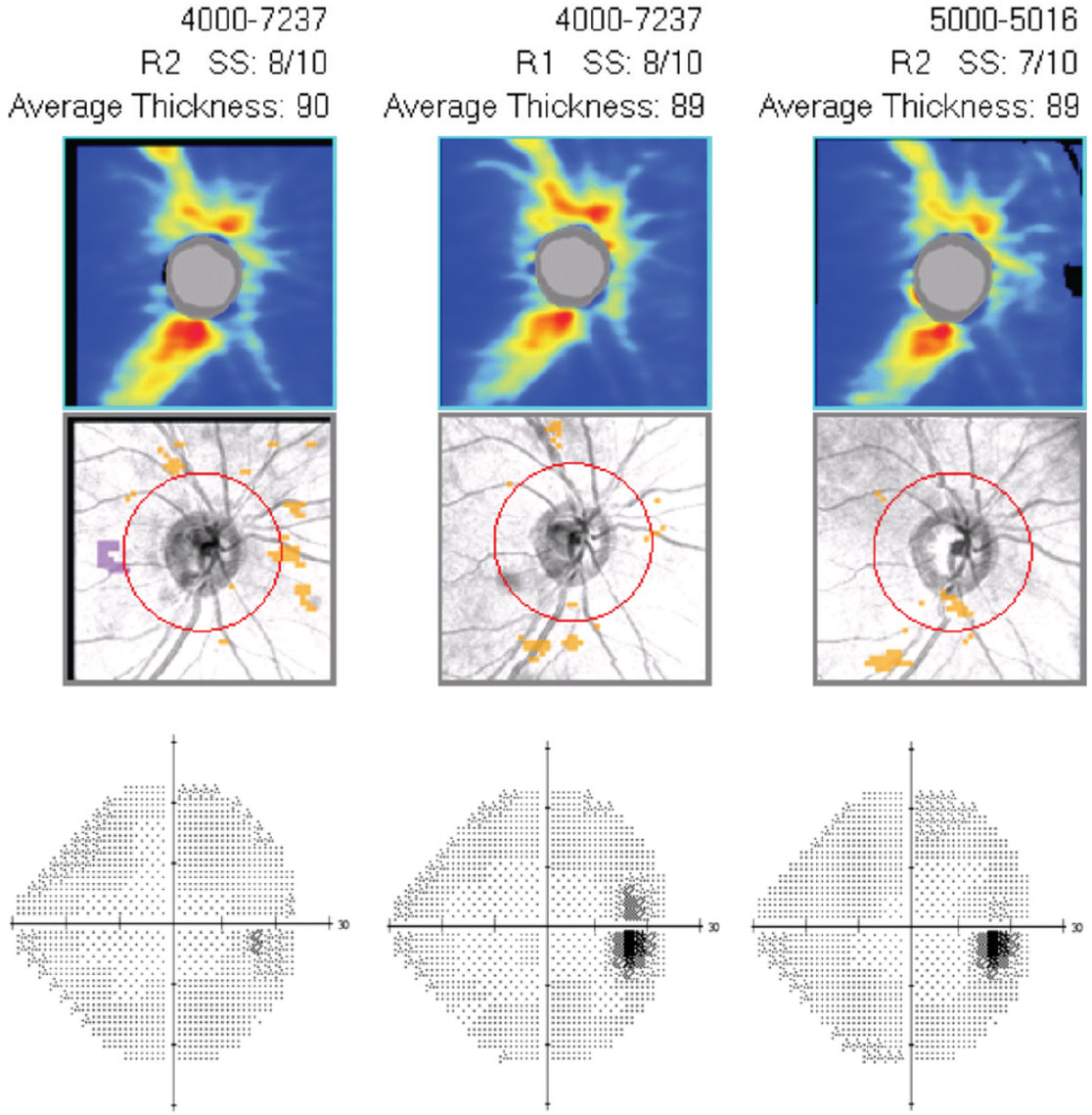 |
| Case 6. This is the guided progression analysis (optic nerve head OCT) with visual fields (right eye only) of a 43-year-old black male who has been treated with Vyzulta for 25 months in both eyes. IOP has decreased 40% and has been consistent at follow ups since initiating the drug. His OCT and visual fields show no change over the 25-month period, indicating successful treatment. Click image to enlarge. |
Glaucoma and Ocular Hypertension
Numerous randomized clinical trials have concluded that lower IOP delays or prevents progression of primary open-angle glaucoma (POAG). The Collaborative Normal Tension Glaucoma Study found slower progression in visual field defects when IOP was lowered by 30% or more.33 A faster rate of progression was noted in women, patients with migraines and those with disc hemorrhages. Notably, the untreated group was highly variable with some patients not progressing in a five-year period.33
In the Ocular Hypertension Treatment Study, researchers found topical ocular hypotensive agents were effective in delaying or preventing onset of POAG in 50% of patients with elevated IOP when IOP was decreased by 20%.34
Management. With the plethora of drug classes available, optometrists can individualize therapy based on drug allergies, systemic medications and ocular diseases. Topical drugs can be classified into three categories based on how they lower IOP: by increasing aqueous outflow via the trabecular meshwork or the uveoscleral meshwork and by decreasing aqueous production.
Within the past two years, two new drugs have come to the market as possible first-line or adjunctive agents for the treatment of glaucoma and OHTN. Vyzulta (latanoprostene bunod 0.024%, Bausch + Lomb) is a dual-mechanism drug that increases uveoscleral outflow of aqueous and increases both trabecular meshwork and Schlemm’s canal outflow via a nitric oxide metabolite.35 In a controlled comparison study of Vyzulta and latanoprost 0.05%, researchers found a statistically significant difference in IOP lowering with Vyzulta at days seven, 14 and 28 compared with latanoprost.35 Vyzulta is dosed one drop at bedtime.
Rhopressa (netarsudil 0.02%, Aerie), another new drug, is a rho kinase inhibitor with three mechanisms to lower IOP: by increasing trabecular meshwork outflow, decreasing aqueous production and lowering the episcleral venous pressure.36,37 In two phase three clinical trials, Rhopressa QD significantly lowered IOP from baseline and was found to be non-inferior to timolol.38
Additionally, in states where laser procedures are included in the optometric scope of practice, selective laser trabeculoplasty (SLT) is an effective first-line therapy when compared with conventional topical medication intervention.39 In one study, first-line SLT treatment provided drop-free IOP control for at least three years and was deemed the most cost-effective first-line therapy for POAG and OHTN.39
Once a patient is diagnosed with treatable glaucomatous disease, clinicians should initiate a topical medication that is most appropriate for the patient and/or discuss SLT treatment. Follow-up is usually six to eight weeks later to determine medication/SLT efficacy (looking for a 20% to 30% decrease in IOP from highest recorded untreated IOP).
At follow up, clinicians should ensure compliance, as prescribed, and any noted side effects that may alter use and compliance. If the efficacy is unsatisfactory, follow up again six to eight weeks later to confirm poor response to the treatment regimen. Yearly structural and functional testing (visual fields and OCT), gonioscopy and optic nerve head photography are important and augment topical treatment with noted progression. With myriad topical drug classes, combinations and laser treatment options, clinicians can delay or adjust topical treatments to meet target IOP while managing practical drop schedules with the patient (Case 6).
If you feel that any presentationis beyond your level of comfort, don’t forget about your colleagues who practice in the various optometric subspecialties who can manage those cases with you. Now more than ever, we are able to provide care to almost all of our patients by implementing the latest technologies and pharmaceuticals to help increase patients’ quality of life through vision.
Dr. Higa is an assistant professor at PCO-Salus, where he works with interns and residents. He has a special interest in ocular surface disease and anterior segment inflammation.
| 1. Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611-9. 2. Coussa RG, Antaki F, Zaguia F, et al. Prognostic factors of postoperative intraretinal cystoid spaces after primary pars plana vitrectomy for vitreomacular traction. J Curr Ophthalmol. 2019;31(4):399-405. 3. Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149(3):371-82. 4. Sebag J. Anomalous posterior vitreous detachment: a unifying concept in vitreo-retinal disease. Graefe’s Arch Clin Exp Ophthalmol. 2004;242(8):690-8. 5. Steel DH, Lotery AJ. Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye. 2013;27(1):S1-21. 6. Jackson TL, Nicod E, Simpson A, et al. Symptomatic vitreomacular adhesion. Retina. 2013;33(8):1503-11. 7. Tadayoni R, Holz FG, Zech C, et al. Assessment of anatomical and functional outcomes with ocriplasmin treatment in patients with vitreomacular traction with or without macular holes: results of OVIID-1 Trial. Retina. 2019;39(12):2341-52. 8. Jackson TL, Nicod E, Angelis A, et al. Pars plana vitrectomy for vitreomacular traction syndrome: a systematic review and metaanalysis of safety and efficacy. Retina. 2013;33(10):2012-7. 9. Irvine SR. A newly defined vitreous syndrome following cataract surgery*: interpreted according to recent concepts of the structure of the vitreous, The Seventh Francis I. Proctor Lecture. Am J Ophthalmol. 1953;36(5):601-19. 10. Gass JD, Norton EW. Follow-up study of cystoid macular edema following cataract extraction. Trans Am Acad Ophthalmol Otolaryngol. 1969;73(4):665. 11. Shoss BL, Tsai LM. Postoperative care in cataract surgery. Curr Opin Ophthalmol. 2013;24(1):66-73. 12. Chu CJ, Johnston RL, Buscombe C, et al; United Kingdom Pseudophakic Macular Edema Study Group. Risk factors and incidence of macular edema after cataract surgery: a database study of 81984 eyes. Ophthalmology. 2016;123(2):316-23. 13. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arteriosclerosis, thrombosis, and Vascular Biology. 2011;31(5):986-1000. 14. Ersoy L, Caramoy A, Ristau T, et al. Aqueous flare is increased in patients with clinically significant cystoid macular oedema after cataract surgery. Br J Ophthalmol. 2013;97(7):862-5. 15. Wielders L, Schouten JSAG, Nuijts RMMA. Prevention and treatment of cystoid macular edema after cataract surgery. Curr Opin Ophthalmol. 2018;29(1):48-53. 16. Comstock TL, DeCory HH. Advances in corticosteroid therapy for ocular inflammation: loteprednol etabonate. Internat J Inflamm. 2012;2012. 17. Sheppard JD, Comstock TL, Cavet ME. Impact of the topical ophthalmic corticosteroid loteprednol etabonate on intraocular pressure. Advances in Therapy. 2016;33(4):532-52. 18. Campochiaro PA, Han YS, Mir TA, et al. Increased frequency of topical steroids provides benefit in patients with recalcitrant postsurgical macular edema. Am J Ophthalmol. 2017;178:163-75. 19. Mastropasqua L, Massaro‐Giordano G, Nubile M, Sacchetti M. Understanding the pathogenesis of neurotrophic keratitis: the role of corneal nerves. J Cell Physiol. 2017;232(4):717-24. 20. Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571. 21. Mackie IA. Neuroparalytic (neurotrophic) keratitis. Presented at the Symposium on Contact Lenses: Transactions of the New Orleans Academy of Ophthalmology. St Louis: Mosby; 1973. 22. Semeraro F, Forbice E, Romano V, et al. Neurotrophic keratitis. Ophthalmologica. 2014;231(4):191-7. 23. Vaidyanathan U, Hopping GC, Liu HY, et al. Persistent corneal epithelial defects: a review article. Med Hypothesis Discov Innov Ophthalmol. 2019;8(3):163. 24. Di Zazzo A, Coassin M, Varacalli G, et al. Neurotrophic keratopathy: pros and cons of current treatments. Ocular Surf. 2019;17(4):619-23. 25. Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye. 2003;17(8):989-95. 26. Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmology. 2020;127(1):14-26. 27. Khokhar S, Natung T, Sony P, et al. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea. 2005;24(6):654-60. 28. Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveillance Summaries. 2017;66(9):1. 29. Pinchefsky E, Dubrovsky AS, Friedman D, Shevell M. Part I—evaluation of pediatric post-traumatic headaches. Ped Neurol. 2015;52(3):263-9. 30. Singman E, Quaid P. Vision Disorders in Mild Traumatic Brain Injury. In: Hoffer M, Balaban C, eds. Neurosensory Disorders in Mild Traumatic Brain Injury. Philadelphia: Academic Press; 2019:223-244. Academic Press. 31. Merezhinskaya N, Mallia RK, Park D, et al. Visual deficits and dysfunctions associated with traumatic brain injury: A systematic review and meta-analysis. Optom Vis Sci. 2019;96(8):542-55. 32. Ciuffreda KJ, Ludlam DP. Conceptual model of optometric vision care in mild traumatic brain injury. J Behav Optom. 2011;22(1):10-3. 33. Group CN. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126(4):487-97. 34. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701-13. 35. Weinreb RN, Ong T, Sforzolini BS, et al. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol. 2015;99(6):738-45. 36. Wang RF, Williamson JE, Kopczynski C, Serle JB. Effect of 0.04% AR-13324, a ROCK, and norepinephrine transporter inhibitor, on aqueous humor dynamics in normotensive monkey eyes. J Glaucoma. 2015;24(1):51-4. 37. Kiel JW, Kopczynski CC. Effect of AR-13324 on episcleral venous pressure in Dutch belted rabbits. J Ocul Pharmacol Ther. 2015;31(3):146-51. 38. Serle JB, Katz LJ, McLaurin E, et al. Two phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure: rho kinase elevated IOP treatment trial 1 and 2 (ROCKET-1 and ROCKET-2). Am J Ophthalmol. 2018;186:116-27. 39. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus drops for newly diagnosed ocular hypertension and glaucoma: the LiGHT RCT. Health Technol Assess. 2019;23(31):1-102. |

