 |
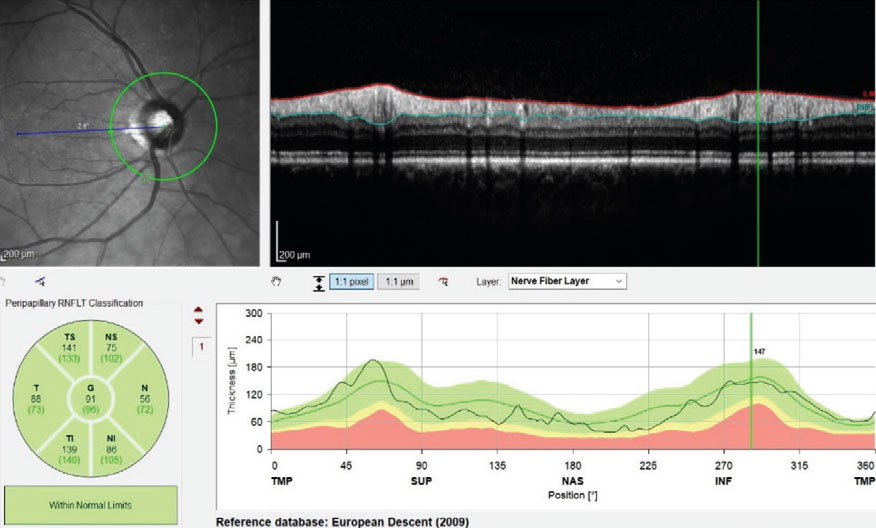
So-called “green disease” misrepresents an eye as normal, concealing pathology due to inadequacies in the reference database. The converse, red disease, suggests disease presence when there is none. A new effort envisions more a robust, real-world database derived from optometric practices that would reduce such errors. Photo: James Fanelli, OD. Click image to enlarge. |
Clinicians rely mostly on summary metrics and the infamous red-yellow-green color coding when using OCT to decide if a patient’s scan is normal or abnormal. Naturally, such analyses are only as good as the databases that comprise the set of ostensibly “normal” eyes used to compare individuals against. That very word has come under fire in recent years as inherently biased and flawed; hence, the eyecare profession has all but dropped mention of “normative” databases in favor of the more neutral term “reference” databases (RDBs).
Still, no matter what you call them, RDBs have three main weaknesses, explains glaucoma expert Michael Chaglasian, OD, one of the researchers of a new study on the topic. First is “small size—about 400 to 600 eyes,” he says, which is the typical number of eyes used in these databases, but not enough to be representative of the wide spectrum of anatomical diversity found in the wider population. Secondly, “only very healthy eyes are included, different from the average patient seen for routine exams.” Finally, “only high quality scans are accepted into the RDB, whereas in regular practice, lower quality scans often have to suffice.” Consequently, he argues, “an OD’s ability to accurately interpret the OCT summary report is confounded and often flawed.”
As such, Dr. Chaglasian and other researchers propose that a larger RDB for OCT would improve the ability of optometrists and ophthalmologists alike to screen for certain diseases like glaucoma. Specifically, Dr. Chaglasian and colleagues—which includes prominent researcher Don Hood, PhD—looked at how feasible it would be to develop a large RDB from OCT scans obtained by optometrists as part of pre-test gathering information, hypothesizing these scans would be sufficient quality to include in a database and would contain lower base rates of glaucoma and other pathologies.
The group reported recently in Optometry & Vision Science on a series of OCT widefield scans obtained from 400 eyes of 400 patients, randomly selected from over 49,000 scans across four optometry sites. Eyes were graded as: unacceptable for RDB use; healthy; optic neuropathy consistent with glaucoma; glaucoma suspect; or other pathology.
Results found 7.3% of scans unacceptable. Of the remaining 371 eyes, 94.9% were graded as healthy. One site did have 7.4% of eyes graded as glaucomatous, but the average of the other three was much lower, at 1.4%. Using reading center criteria for adjustments, the team opted to exclude over half of scan deemed either glaucomatous or representing other pathology.
The study authors relay the implications of these results for a real-world reference database, which consists of making decisions of what constitutes the definition of “acceptable scan quality” and “healthy eyes.”
Removing unacceptable scans is typically done at levels of the technician, testing site and in independent reading centers to determine a definition of scan quality, excluding for instances based on image quality score, presence of eye blinks, eye motion, clipping, local weak signal and feature centration; the only objective measure is image quality index. This study’s approach was similar, discarding scans with evident errors, but specifying that these errors would be artifacts that would affect circumpapillary retinal nerve fiber layer thickness (cpRNFL) and/or ganglion cell layer (GCL) thickness measures, which are the most common summary metrics used. However, they make note that when assessing for exclusions, it is important not to eliminate OCT scans that represent the low end of normal of RNFL and GCL thicknesses, as doing so would result in worse specificity, which was the case of the commercial RDB when assessed with using real-world healthy eyes.
All in all, the effort produced encouraging results. “The scans from optometry practices that are primarily involved in refraction and medical screening services should yield a large, real-world RDB with improved specificity and a base rate of glaucoma and/or other pathologies comparable to existing RDB,” the team noted in their paper on the work.
The authors argue that three strategies can be used to create a real-world reference database from optometric practices’ scans to minimize how many glaucomatous and other pathologic eyes are included. One is to have a reading center perform complete reviews of all scans, an impractical idea. The other two options take less time: (1) to include optometry sites mostly involved in refraction and medical screening services, or (2) to expand the ‘unacceptable’ category to include eyes with incomplete circles of GCL+ thickness (eyes with glaucomatous or other retinal loss) and not just those with artifacts.
Though no formal effort is on the drawing boards yet, the present work seems to lay the foundation for a proposal to develop a new and more representative reference database derived from optometric offices across the United States.
Dr. Chaglasian expands on the research’s likely direction, pointing out that “potentially, a much, much larger database would improve the specificity, while maintaining high sensitivity. The larger sample size will include many of the common, non-disease anomalies, that are seen in general practice on mostly healthy eyes. The software analysis of the identified normal (green), vs. borderline (yellow) vs. abnormal (red), will then be better able to make these differentiations.”
Hood DC, Durbin M, Lee C, et al. Towards a real-world optical coherence tomography reference database: optometric practices as a source of healthy eyes. Optom Vis Sci. 2023. [Epub ahead of print]. |

