 |
A 42-year-old Hispanic woman presented to our institute with three weeks of painless blurry vision in the left eye. Past medical history was positive for gastroesophageal reflex disease that was managed medically, past ocular history was unremarkable, family history was negative for eye-related diseases and this was her first eye exam.
Entrance visual acuities were 20/20 in the right eye and 20/80 with pinhole improvement to 20/70 in the left eye. Intraocular pressure was 18mm Hg OD and 19mm Hg OS, extraocular motilities were full in all gazes and there was a relative afferent pupillary defect OS. Anterior segment examination was normal and posterior segment imaging is available below for review.
Take the Retina Quiz
1. The fundus imaging of the left eye shows which of the following?
a. Orange pigment on clinical exam corresponding with hyperfluorescence at the nasal margin of the macular lesion.
b. Subretinal fluid on OCT.
c. Dome-shaped acoustically hollow lesion on B-scan.
d. All of the above.
2. What is the most likely diagnosis?
a. Choroidal hemangioma.
b. Choroidal melanoma.
c. Choroidal nevus.
d. Peripheral exudative hemorrhagic chorioretinopathy.
3. Which of the following clinical features increases risk of metastasis?
a. Increasing thickness.
b. Presence of ciliary body extension.
c. Presence of extraocular extension.
d. All of the above.
4. Which of the following statements regarding intervention is most appropriate?
a. This lesion looks benign and should be observed without treatment.
b. This lesion looks suspicious and should be monitored with repeat examination in six months.
c. This lesion is too large for treatment and should undergo primary enucleation.
d. This lesion should undergo plaque brachytherapy with or without fine needle aspiration biopsy.
5. Which of the following statements is FALSE?
a. Preferentially expressed antigen in melanoma (PRAME)-positive lesions carry an increased risk of metastasis vs. PRAME-negative.
b. Gene expression profiling (GEP) class 1A is the highest risk class for metastasis.
c. BAP1 mutation carries risk of malignant neoplasm formation and metastasis.
d. All of the above are true.
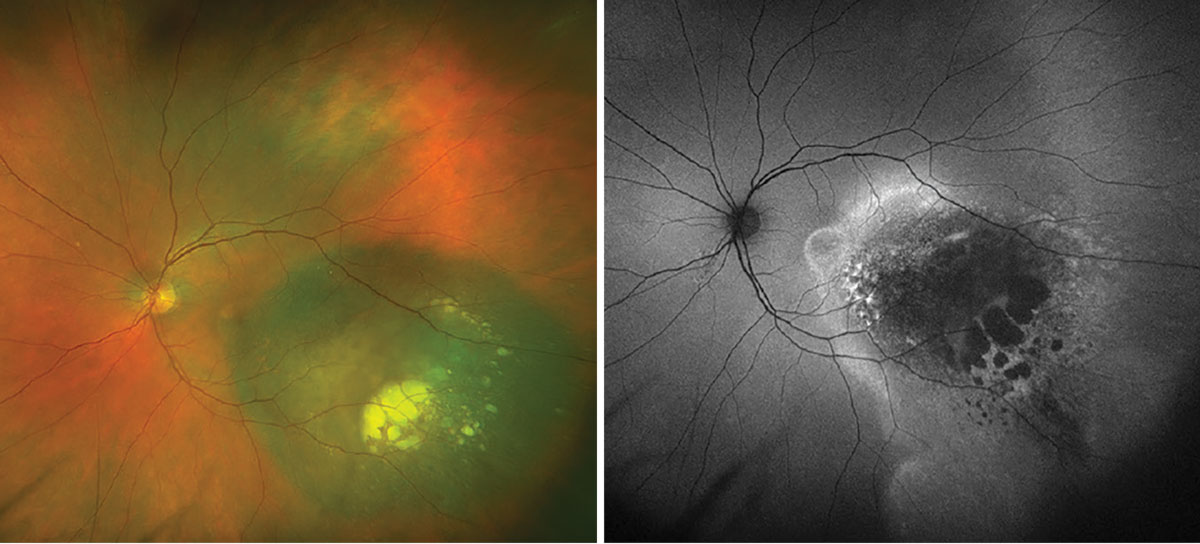 |
Fig. 1. Optos widefield fundus photography and autofluorescence of the left eye. Click image to enlarge. |
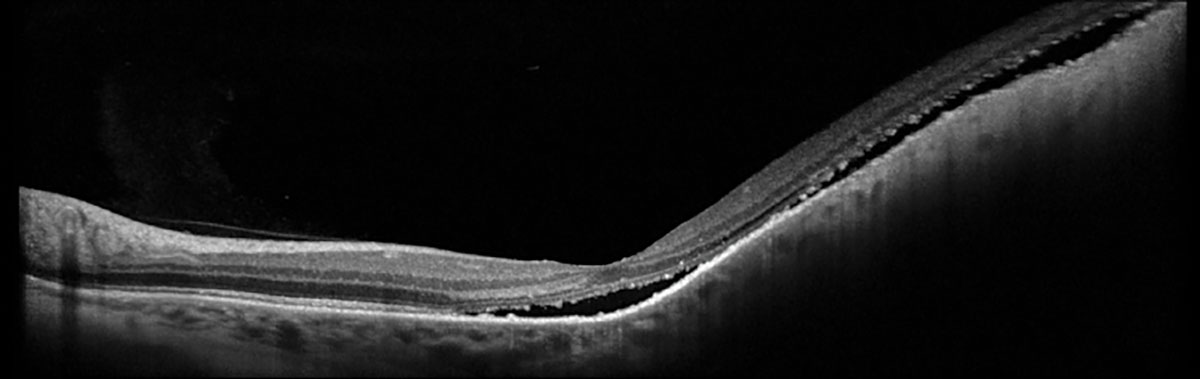 |
Fig. 2. Heidelberg OCT of left eye. Click image to enlarge. |
Diagnosis
Fundus examination and ophthalmic imaging of the right eye was normal. Examination of the left eye revealed a round, elevated, hyperpigmented melanocytic lesion with lipofuscin at the nasal margin as well as atrophy, drusenoid deposits and exudative material at the temporal margin (Figure 1A). Fundus autofluorescence (FAF) showed hyperAF corresponding with the lipofuscin, hypoAF corresponding with the atrophy and a hyperAF trail guttering inferiorly corresponding with the chronic exudative retinal detachment (Figure 1B). OCT depicted a large, elevated, dome-shaped choroidal lesion with subretinal fluid involving the fovea (Figure 2). B-scan ultrasonography demonstrated an acoustically hollow dome-shaped lesion with basal diameter of 12.1mm x 12.7mm and maximal thickness of 2.7mm (Figure 3).
Clinical examination and ophthalmic imaging were consistent with a diagnosis of posterior uveal melanoma. Given the new diagnosis, the patient was screened for metastatic disease with a computed tomography (CT) scan of the chest, abdomen and pelvis with and without contrast which showed no evidence of metastases. The patient underwent radioactive I-125 plaque brachytherapy and prognostic fine needle aspiration biopsy (FNAB) of the tumor.
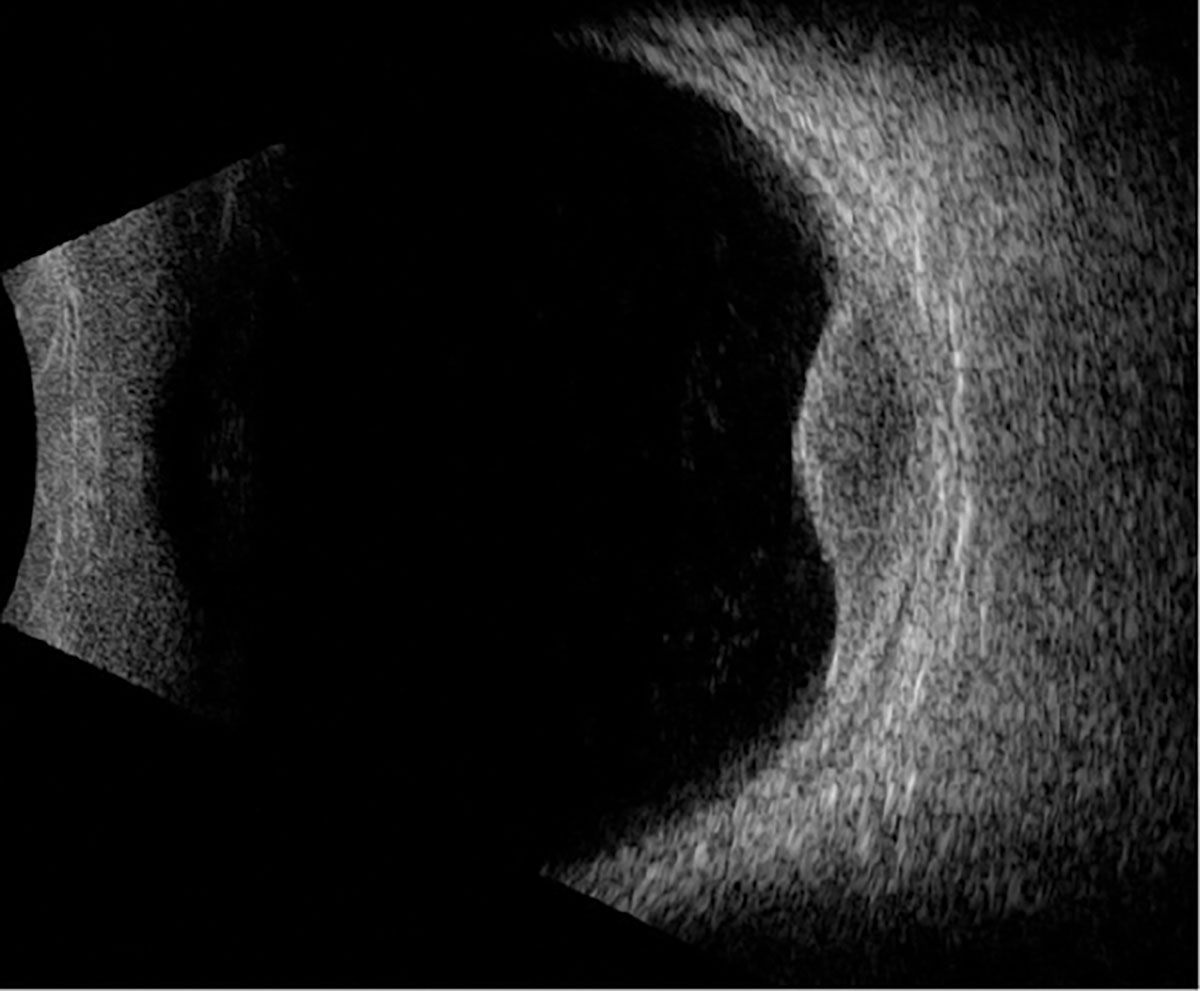 |
Fig. 3. B-scan ultrasound through the lesion. Click image to enlarge. |
Uveal melanoma (UM) is a malignant neoplasm of the uveal tract. It is the most common noncutaneous melanoma and primary intraocular malignancy in adults.1,2 There are about 7,000 new cases annually: 90% involve the choroid, 6% involve the ciliary body and 4% involve iris.1,2
About 80% of UM cases occur in patients between 45 to 60 years of age (mean age 60 to 65 in the US).2 Ethnic distribution of incidence is 0.3, 0.2, 1.7 and 6.0 cases per million among Africans, Asians, Hispanics and non-Hispanic Caucasians, respectively.2,3 Among non-Hispanic Caucasians, incidence ranges from 2.6 in Southern US and Europe to as high as 8.4 cases per million in Northern US and Scandinavia.2,4
Melanin is thought to be protective against tumor formation.5 Risk factors for developing UM include fair skin complexion, light-colored iris, inability to tan, choroidal nevus, ocular or oculodermal melanocytosis, dysplastic nevus syndrome and genetic predisposition (e.g., mutation in the BRCA1-associated protein-1; BAP1).2,4 BAP1 gene mutations carry an increased risk of developing cancers of the eye, kidney, skin and mesothelium, as well as for metastatic disease.4
UM may be described as being anterior (involving iris) or posterior (involving the ciliary body and choroid).1,4 The current convention for classification of posterior UM was updated in 2013 to address primary tumor anatomic features (T), regional lymph node metastasis (N) and systemic metastasis (M).2,6 The lesions are first classified as small (T1), medium (T2), large (T3) or very large (T4) based on lesion thickness and basal diameter, then further stratified into one of seven possible stages (I, IIA, IIB, IIIA-C and IV) based on presence of ciliary body and/or extrascleral extension.1,2,6
The differential diagnosis includes choroidal nevus, choroidal hemangioma, peripheral exudative hemorrhagic chorioretinopathy, congenital hypertrophy of the retinal pigment epithelium (RPE), or subretinal/sub-RPE hemorrhage.4 Enhanced-depth imaging OCT can characterize smaller, posterior lesions, but ultrasonography remains the most important diagnostic tool.2
A-scan features include low amplitude internal reflections with occasional spikes that may represent intrinsic tumor vascular pulsations.2
Typical B-scan features include acoustic hollowing, choroidal excavation and orbital shadowing.1,2,4 Once Bruch’s membrane has been breached, the lesion takes on a typical “collar-button” or “mushroom” configuration on ultrasound and may show the characteristic “double circulation” on fluorescein angiography due to intrinsic tumor vascularity overlying the highly vascularized choroid.1,2,4
 |
|
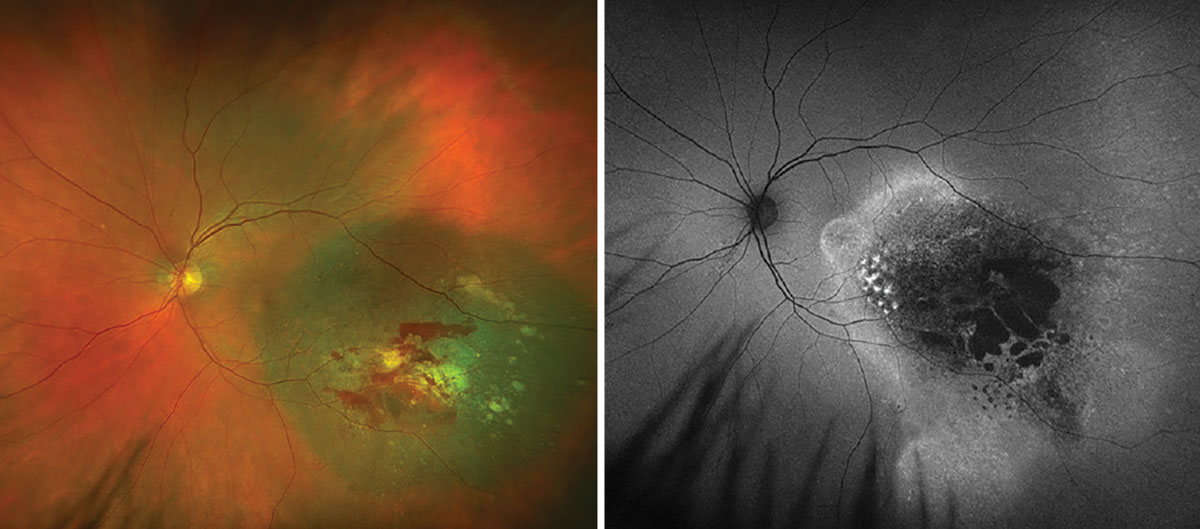
Fig. 4. Optos widefield fundus photography and autofluorescence of the left eye at one-month post-op. Click image to enlarge. |
Management
Small (8mm to 10mm basal diameter and <3mm apical thickness) posterior UM can be carefully observed by an ocular oncologist if lesions are stable/slow-growing or when risks outweigh the benefit of treatment.1 When treatment is indicated, transpupillary thermotherapy (TTT) has been employed as a method of inducing immediate tumor necrosis for pigmented tumors <4mm thick with a recurrence rate of 9% to 28%.4 Small, amelanotic tumors are best treated with photodynamic therapy with a recurrence rate of approximately 21%.4
Medium-sjze tumors (<16mm basal diameter and 3mm to 10mm apical thickness) are generally managed with plaque radiotherapy, which is equally as effective as enucleation, proton beam and stereotactic radiation.4 Iodine-125 is the most commonly used radioisotope within plaques and can achieve excellent tumor control with only 3% recurrence when combined with TTT.4 Large tumors (basal diameter >18mm, thickness >12mm), eyes with poor visual potential, and those with moderate extraocular extension may be recommended enucleation and cases of extraocular extension into the orbit may require exenteration.4
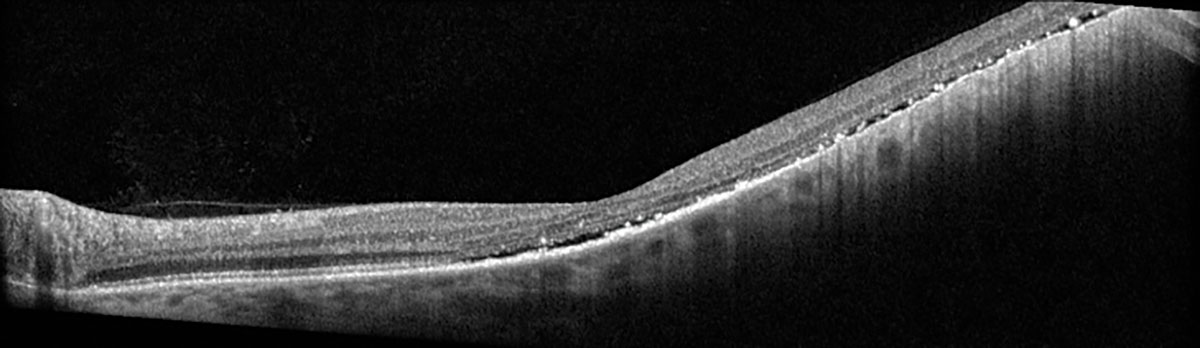 |
Fig. 5. Heidelberg OCT of the left eye at one-month post-op. Click image to enlarge. |
Prognosis
While metastases at the time of UM diagnosis are rare (<2%), all patients must undergo initial systemic screening with CT or MRI of the chest, abdomen and pelvis with subsequent lifelong surveillance.2,4 It is important to rule out metastatic disease as well as any additional primary cancers, present in up to 10% of patients.2,4 Liver is the most common site of metastasis (100% of patients with metastatic disease), followed by lungs.2,4
The American Joint Committee on Cancer stratified metastatic risk based on tumor basal diameter, with 10-year metastatic risks of 6%, 20%, 32% and 47% for T1, T2, T3 and T4 sizes, respectively.7 Ciliary body and extraocular extension portend much greater chance of metastasis (67% at 10 years).7,8 The 10-year survival rates range from 88% to 94% for stage I tumors, 80% to 84% for stage IIA, 67% to 70% for stage IIB, 45% to 60% for stage IIIA, 27% to 50% for stage IIIB, 0 to 10% for stage IIIC and <1% for stage IV.7,8 This corresponds to a twofold increased metastatic risk for each increase in stage.7
FNAB can identify tumor molecular class for additional metastatic prognostication; GEP can further divide UM into class 1A (low risk), class 1B (intermediate risk) and class 2 (high risk). The five-year rate of metastasis is estimated to be 2% for class 1A, 21% for class 1B and 72% for class 2.9 Additionally, tumor expression of PRAME is thought to be a predictor for increased likelihood of metastasis in class 1 tumors and shorter duration to metastasis in class 2 tumors.10,11
Patient Management
FNAB was performed on our patient at the time of uncomplicated plaque radiotherapy placement. Cytogenetics revealed that the tumor was GEP class 1A and PRAME negative, allowing for a tailored discussion regarding her more benign prognosis with the lowest likelihood of metastasis. At one-month post-op, there was mild subretinal hemorrhage, stable FAF and improvement in the subretinal fluid (Figures 4 and 5). She continues to be followed to monitor tumor response and will undergo routine surveillance with liver function studies and systemic imaging annually.
Retina Quiz Answers
1: d, 2: b, 3: d, 4: d, 5: b
Dr. Aboumourad currently practices at Bascom Palmer Eye Institute in Miami. He has no financial disclosures.
1. Scott JF, Gerstenblith MR. Noncutaneous ,elanoma. Codon Publications; 2018. 2. Syed N, Berry J, Heegaard S, et al. 2020-2021 BCSC: Basic and clinical science course. American Academy of Ophthalmology; 2019. 3. Kivelä T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93(9):1129-31. 4. Kaliki S, Shields CL. Uveal melanoma: relatively rare but deadly cancer. Eye (Lond). 2017;31(2):241-57. 5. Shah CP, Weis E, Lajous M, et al. Intermittent and chronic ultraviolet light exposure and uveal melanoma: a meta-analysis. Ophthalmology. 2005;112(9):1599-607. 6. Kujala E, Damato B, Coupland SE, et al. Staging of ciliary body and choroidal melanomas based on anatomic extent. J Clin Oncol. 2013;31(22):2825-31. 7. AJCC Ophthlamic Oncology Task Force. International validation of the American Joint Committee on Cancer’s 7th edition classification of uveal melanoma. JAMA Ophthalmol. 2015;133(4):376-83. 8. Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44(11):4651-9. 9. Ballhausen A, Urias E, Gruschkus SK, et al. Metastatic risk factors associated with class 1A uveal melanoma patients. Cancers (Basel). 2021;13(13):3292. 10. Epping MT, Bernards R. A causal role for the human tumor antigen preferentially expressed antigen of melanoma in cancer. Cancer Res. 2006;66(22):10639-42. 11. Field MG, Decatur CL, Kurtenbach S, et al. PRAME as an independent biomarker for metastasis in uveal melanoma. Clin Cancer Res. 2016;22(5):1234-42. |

