
In the late 19th century, Alexios Trantas was the first person to describe the anterior drainage angle.1 The two-step method we use to view this structure today, gonioscopy, has remained relatively unchanged since the mid-20th century. The first step: Perform the exam. The second: Interpret the results. As with any procedure, proficiency comes with experience. Gonioscopic examination of healthy patients allows you to learn the variants of the typical angle, and time spent with a mentor can also be very beneficial.
Despite its long tradition, this procedure continues to be underutilized. Recent studies show that only about 50% of glaucoma patients receive a gonioscopic exam.2,3 Indications for gonioscopy include glaucoma or suspicion of glaucoma, narrow anatomical angles, risk for angle neovascularization and a history of ocular trauma.
Gonioscopy is first used to detect occludable vs. open angles. Second, gonioscopy is used to detect abnormal angles, such as those seen in cases of Axenfeld-Reiger syndrome, pigment dispersion syndrome, exfoliation syndrome, neovascularization, hyphema, angle recession, iridodialysis, peripheral anterior synechiae (PAS) and iridocorneal endothelial syndromes.
When gonioscopy is indicated, it should be performed after applanation tonometry in order to ensure that the IOP is not artificially modified due to pressure applied during the procedure.
Performing Gonioscopy
Most clinicians perform indirect gonioscopy with a three- or four-mirror gonioprism (with or without a flange). The three-mirror and four-mirror prisms, when used with a flange, couple with the cornea and are stable on the eye, but they do not allow for indentation gonioscopy. The four-mirror prism without the flange is quick and allows for indentation gonioscopy, but is less stable. A three-mirror lens is the slowest to use, but it offers the highest magnification, which makes it the best choice when evaluating for neovascularization of the angle.
In order to perform gonioscopy, necessary supplies include a cushioning solution, an anesthetic without sodium fluoride and a clean gonioscopy lens. If you use a three-mirror lens, view the drainage angle with the thumbnail-shaped mirror. Place the appropriate mirror 180 across from the angle that you wish to viewed. With gonioscopy lenses, rotation of the lens is needed in order to view all the drainage angles. A smooth, even pressure should be applied to the lens on the cushioning solution, and take care not to push too much.
Indentation gonioscopy is when you exert force with a gonioprism with a smaller surface area than the cornea and mechanically open the angle. It is extremely useful when viewing around a steep iris approach and when differentiating appositional vs. synechial closure. But, indentation gonioscopy is somewhat controversial. If the angle is artificially opened with indentation, it may remain occludable. While we believe this may be an immoderate view, most practitioners would agree that indentation gonioscopy not only artificially opens the angle, but also allows for viewing of structures that are unable to be seen otherwise. Another consideration is that slit lamp-induced miosis may artificially overstate the angle; so, reduce ambient room lighting, and do not let the beam enter the pupil while performing the exam. Plateau iris syndrome will form characteristic peripheral iris folds with indentation.
Normal Drainage Angle Structures
The normal angle structures of an open angle, as shown in figure 1, are the iris, ciliary body band, scleral spur, trabecular meshwork and Schwalbes line (from posterior to anterior). The iris attaches to the ciliary body band in an open angle. Iris processes (figure 2) may be present and are normal extensions of iris tissue into the scleral spur. The ciliary body band varies in color from pinkish, to light or dark brown, to slate gray.

1. A grade 4 open angle. Note the iris, ciliary body band, scleral spur trabecular meshwork and Schwalbes line.
The scleral spur is the next normal angle structure. It is the site of attachment of the longitudinal muscle of the ciliary body and is the anterior extension of the sclera. The scleral spur appears as a narrow, white band and is relatively consistent between patients. Following the scleral spur anteriorly, the trabecular meshwork is separated into two components: posterior and anterior. The posterior trabecular meshwork is pigmented, and the anterior trabecular meshwork ranges from light brown to white. Blood in Schlemms canal (within the trabecular meshwork), induced by pressure on gonioscopy, can locate a minimally pigmented trabecular meshwork.
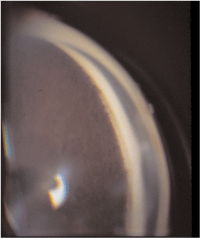
2. A grade 3 angle with visible iris processes.
The final normal structure visualized in an open drainage angle is Schwalbes line, a shelf-like component created by the termination of Descemets membrane that is comprised of a circumferential ring
of collagen fibers. Generally, Schwalbes line is the most difficult structure to visualize during gonioscopy, but it may appear as a bright white line anterior to the trabecular meshwork. To assist in locating Schwalbes line, you can look to find the point of the junction created by the convergence of the anterior and posterior corneal reflections (corneal wedge) into a single reflection in the angle.
When grading the drainage angle, you should note any pigmentation within the angle. Excessive pigmentation may suggest a pathologic condition, while mild pigmentation within the angle may be normal and due to pigment liberation. The approach of the iris to the drainage angle should be described as well. The iris may be regular, steep (bowing forward toward the cornea), or posteriorly bowed (also described in some grading systems as queer).
There are a number of grading techniques and protocols used to evaluate the drainage angle. The most straightforward system is to document: 1) the most posterior angle structure in each quadrant; 2) the iris approach; 3) the trabecular meshwork pigmentation; and 4) pertinent negatives of PAS, angle recession and neovascularization of the angle.
Anomalies of the Drainage Angle
Angle closure. Angle closure may be chronic, intermittent or acute. Patients with angle closure demonstrate a variety of ocular findings, including an occluded (acute) or occludable angle (figure 3). The non-angle ocular signs include corneal edema and conjunctival redness that may be significant, with severe symptoms, including pain, cloudy vision and halos around lights, not to mention nausea.

3. A grade 1 occludable angle.
When gonioscopy is performed, no angle structuresor only Schwalbes linewill be visible during an angle closure attack. Acute angle closure may allow for indentation because the angle is not synechially closed. Indentation gonioscopy may assist in lowering the IOP during an acute attack, and it indicates that laser peripheral iridotomy (LPI) may be an effective treatment. Intermittent angle closure may result in PAS, foggy vision and painful episodes with conjunctival redness that resolve without management. Chronic angle closure will result in multiple PAS that limit indentation gonioscopy and the efficacy of laser peripheral iridotomy, but it may not present with obvious physical signs, such as redness.
Axenfeld-Reiger syndrome. The congenital anterior segment disorders, traditionally referred to as the angle cleavage syndromes, and now referred to collectively as Axenfeld-Reiger (A-R) syndrome, represent a continuum of disease. These disorders are bilateral with variable penetrance, they may be asymmetric, and they are generally autosomal dominant.4 One newer theory on A-R pathogenesis suggests a developmental arrest of specific anterior segment tissues derived from neural crest cells, which results in the retention of a primordial endothelial cell layer on portions of the iris and angle structures, forming a membrane of sorts.5 Over time, there is a contraction of these endothelial membranes that lead to the associated ocular anomalies along the spectrum of the syndrome.
Throughout the spectrum of the A-R syndrome disorder, all stages of the condition present with a prominent anteriorly displaced Schwalbes line (posterior embryotoxin). Other associated ocular findings that define the spectrum of the disease include iris strands to Schwalbes line, iris hypoplasia, focal iris atrophy, corectopia and ectropian uveae. Additionally, non-ocular associations range from variations in teeth, facial bones, pituitary anomalies and heart disease to oculocutaneous albinism. Patients with ocular anomalies are generally asymptomatic and diagnosed through routine biomicroscopy and gonioscopic evaluation. Symptoms may follow any uncontrolled glaucoma.

4. In Axenfelds anomaly, posterior embrotoxin is associated with prominent iris processes.
The risk of glaucoma is approximately 50% within the A-R spectrum.6 Axenfelds anomaly (figure 4) is identified when posterior embryotoxin is accompanied by prominent iris processes. In cases of Alagilles syndromeAxenfelds anomaly associated with pigmentary retinopathy, corectopia, esotropia, and systemic anomaliespatients demonstrate abnormal electroretinogram and electrooculogram results. The condition has been mapped to multiple gene areas.7
Another variation within the A-R spectrum is Riegers anomaly, which occurs with all of the findings of Axenfelds anomaly as well as iris hypoplasia with holes. Riegers syndrome is an autosomal-dominant pathology that combines Riegers anomaly with mental developmental delay and dental, craniofacial, genitourinary and skeletal abnormalities.
Both types of Peters anomaly are more rare A-R variants, in which only 80% of cases present bilaterally.8 The ocular findings noted with this presentation include corneal leukoma due to a central defect of Descemets membrane and an endothelium with iris adhesions. Peters anomaly may present either with or without cataract.
Pigment dispersion syndrome. The most common drainage angle characteristic associated with pigment dispersion syndrome (PDS) is excessive, released iris pigment that has settled into the trabecular meshwork.
Patients with more significant pigment release have a greater amount of trabecular meshwork pigmentation, which results in a darker appearance. When the trabecular meshwork pigment is dense and continuous, the resulting gonioscopic finding may be referred to as a chocolate line (figure 5). Other pigment may be seen settling onto and anterior to Schwalbes linea clinical presentation called Sampaolesis line.
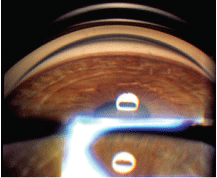
5. In pigment dispersion syndrome, a chocolate line is often visible on gonioscopy.
Another finding often viewed gonioscopically in patients with PDS is a posterior bowed (queer) iris. In patients with the most common variety of PDS, the posterior bowed iris rubs in the midperiphery against the lens zonules.
Exfoliation syndrome (figure 6). Also known as pseudo-exfoliation syndrome, exfoliation syndrome (EXS) presents with pigment in the drainage angle and possibly visible exfoliative material settled within the trabecular meshwork. Unlike the presentation of PDS, pigment found in patients with EXS is not as dense or evenly distributed. Rather, the splotchy presentation of pigment in EXS may be accompanied by fluffy, white material, which comes from the abnormal basement membrane deposition that also can be seen on the lens. The exfoliative material further disrupts outflow through the drainage angle and may contribute to the increased IOP.
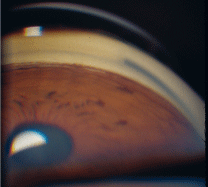
6. Exfoliation syndrome presents with pigment in the angle and possibly exfoliative material in the trabecular meshwork.
Neovascularization (figure 7). Neovascularization of the angle (NVA) may be secondary to iris neovascularization (NVI), or it may be the initial location of new blood vessel growth, depending on the site of ocular ischemia. The vessels associated with NVI are especially fine and delicate in comparison to normal iris vessels. Also, angle neovascularization does not follow the normal anatomical direction of vessels within the iris (neovascularization generally follows the areas of least resistance), and it may be connected to areas of NVI, which can start from the pupillary margin.

7. Neovascularization of the angle may be secondary to iris neovascularization.
Initially, when glaucoma associated with NVA occurs, it is a type of open-angle glaucoma. As the neovascularization progresses along the drainage angle, however, it functions in a zipper-like action, closing the angle. A further ocular complication that can result from NVA is hyphema.
Trauma. Ocular trauma can also result in hyphema, as well as a variety of other drainage angle findings. When hyphema is associated with trauma, there is a high risk of angle recession.9 If you suspect angle recession, perform gonioscopy on the fellow eye firstthis way, you can observe the patients normal ocular anatomy. Looking at an eye with angle recession, large or small sections of the ciliary body may be markedly exposed (figure 8).
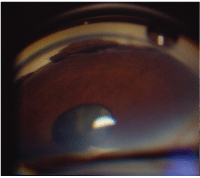
8. In angle recession, sections of the ciliary body may be markedly exposed.
Depending on the nature of the injury, another ocular finding in patients who have suffered trauma may be excessive pigment within the drainage angle. Pigment may be especially prevalent when there is blunt force trauma to the eye. Moreover, when a patient has ocular trauma (including surgery) that results in severe or longstanding uveitis, he or she may develop peripheral anterior synechiae (PAS). These appear as tent-like elevations of the anterior iris surface to structures within the drainage angle or the peripheral cornea (figure 9). As opposed to iris processes, PAS are distinct physical barriers to aqueous outflow that frequently produce increased IOP and cause further complications in treatment and management.
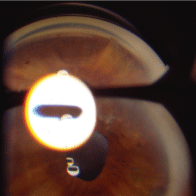
9. Peripheral anterior synechiae appear as elevations of the anterior iris surface to structures within the drainage angle or peripheral cornea.
Chronic inflammation. Systemic conditions that cause chronic uveitis may also cause PAS. When the eye has long periods of active inflammation with cells and flare, the inflammation leads to the connection of iris tissue and other anterior structures such as those in the drainage angle. Systemic conditions that produce chronic uveitis include (but are not limited to) sarcoidosis, systemic lupus erythematosus and various arthritides. Regular gonioscopic evaluations on patients with chronic uveitis is essential; changes if IOP may be attributed to a variety of associated entities, including steroid response or uveitic glaucoma, which could lead the practitioner to overlook the development of PAS.
If PAS are overlooked, management may be compromised; some medications may not be as affective and surgical options may become limited.
Iridocorneal endothelial syndromes. Iridocorneal endothelial (ICE) syndromes occur in young to middle-aged adults, and in women more than men. This unilateral condition is a result of an abnormal corneal endothelium, which causes corneal edema, iris changes and progressive angle closure. Early in the disease, patients are usually asymptomatic or may notice only slight iris irregularities. As the condition develops, though, the patient may begin to experience decreased vision and pain secondary to corneal edema. Later, the patient may present with significant pain and corneal edema associated with increased IOP.
10.
The three variations of the ICE syndromes are essential iris atrophy,
The ICE syndromes may lead to increased IOP and may demonstrate peripheral anterior synechiae that extend to or beyond Schwalbes line as well as a secondary cellular membrane that extends over the drainage angle and PAS. Contraction of the membrane creates PAS and results in corectopia. The iris atrophy may worsen to hole formation, and iris nodules may occur in the area of the membrane.
Gonioscopy Findings and Associated Conditions
Finding
Pigment
Peripheral anterior synechiae
Hyphema
Exfoliative material
Wide ciliary band
Blood vessels
Iris processes
Associated Condition(s)
Pigmentary dispersion syndrome, exfoliation
syndrome, trauma and surgery
Intermittent or chronic angle closure attacks,
trauma and chronic inflammation
Neovascularization of the angle, trauma
Exfoliation syndrome
Angle recession
Neovascularization, normal iris vessels
Normal finding, Axenfeld-Reiger syndrome
To most effectively manage patients, perform gonioscopy when indicated and clearly document all findings. This procedure is essential in making management choices for patientsespecially in anticipating the effectiveness of medications versus surgical options in the treatment of glaucoma. By effectively recognizing drainage angle anomalies, optometric physicians can provide more timely care for patients at risk for vision loss and further understand the potential for progression of some disease entities.
Dr. Black is an assistant professor at Nova Southeastern University College of Optometry and chief of service at Fort Lauderdale Clinic in
1. Dellaporta A. Historical notes on gonioscopy. Surv Ophthalmol 1975;20(2):137-49.
2. Quigley HA, Friedman DS, Hahn SR. Evaluation of practice patterns for the care of open-angle glaucoma compared with claims data: the Glaucoma Adherence and Persistency Study. Ophthalmology 2007 Sep;114(9): 1599-606.
3. Coleman
4. Gould DB, Mears AJ, Pearce G, Walter MA. Autosomal dominant Axenfeld-Rieger anomaly maps to 6p25. Am J Hum Genet 1997;61:765-8.
5. Shields, MB. Axenfeld-Rieger syndrome: A theory of mechanism and distinctions from the iridocorneal endothelial syndrome. Tr Ann Ophth Soc 1983;81:736-84.
6. Schoneveld PG, Pesudovs K. Iridoschisis. Clin Exp Optom 1999;82(1):29-33.
7. Krantz ID, Colliton RP, Genin A, et al. Spectrum and frequency of jagged1 (JAG1) mutations in Alagille syndrome patients and their families. Am J Hum Genet 1998 Jun;62(6):1361-9.
8. Traboulsi EI, Maumenee IH. Peters anomaly and associated congenital malformations. Arch Ophthalmol 1992 Dec;110(12):1739-42.
9. Sihota R, Kumar S, Gupta V, et al. Early predictors of traumatic glaucoma after closed globe injury: trabecular pigmentation, widened angle recess, and higher baseline intraocular pressure. Arch Ophthalmol 2008 Jul;126(7):921-6.

