Glaucoma is a pressing public health concern, specifically due to the asymptomatic nature of the disease, the increasing prevalence and the risk for blindness. Researchers estimate that approximately 2% of the US population aged 40 to 80 years have a diagnosis of glaucoma, with another 2% undiagnosed.1 Furthermore, studies estimate that the number of people with glaucoma in the United States is expected to increase to 6.3 million by 2050 and to 112 million worldwide by 2040.2,3
With glaucoma on the rise, clinicians need to be prepared to diagnose these patients early and choose the best treatment plan. But making a diagnosis of glaucoma can be challenging—not only because glaucoma itself is complex and involves many variables, but also because many other pathologies can masquerade as glaucoma. This article discusses the various differential diagnoses to help clinicians properly identify the true disease process in play and plan a management strategy accordingly.
POAG vs. Secondary
First and foremost, practitioners must differentiate primary open angle glaucoma (POAG) from secondary glaucoma, as the different pathophysiology of the glaucomatous damage will dictate the first line of treatment.
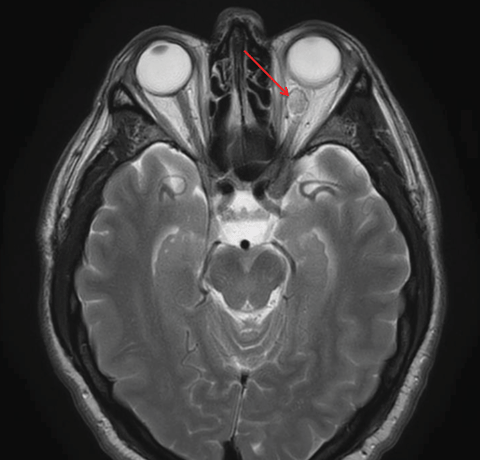 |
| This patient’s axial MRI shows a left intraconal lesion of orbit compressing the optic nerve. |
Patient demographics are also important, as POAG is found more frequently in the older population. If a patient presents with signs consistent with glaucoma, is below the age of 40 and shows no signs of a secondary glaucomatous process, other diagnoses should be considered. In a review study that looked at primary open angle glaucoma prevalence across different ethnicities, researchers found that, most often, glaucoma prevalence was low below the age of 40, at about 2% to 3% across all ethnicities. The same study found that Americans of African decent had the highest prevalence, about 5%, at 60 years of age. The greatest increase of prevalence with age was in Hispanics and Caucasians.2 Therefore, it is vital for clinicians to be cognizant of the red flags that may elicit an alternative diagnosis.
Once clinicians have differentiated primary from secondary glaucoma, we must gain a firm understanding of the patient’s systemic health through a thorough examination, rule out therapeutic contraindications and tailor the topical therapy to best suit the initial target range. Clinicians must take into consideration ease of use, possible side effects, cost and the medication’s mechanism of action to determine the best topical management for each patient.
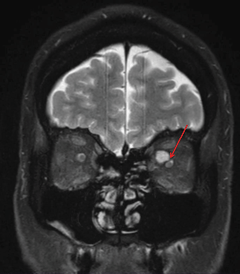 |
| The same patient’s coronal MRI shows the compression of the ONH by orbital lesion. |
Glaucoma Masqueraders
Common masqueraders of glaucomatous optic neuropathy are usually ocular conditions or manifestations of ocular disease that can change the appearance of the optic nerve. Studies have shown that particular exam testing—specifically, visual acuity, visual field testing and serial retinal photography—can help differentiate between nonglaucomatous optic nerves and glaucomatous ones. As a primary eye care provider, recognizing a variance in the disease course is essential in properly taking care of patients. Clinicians should be on the lookout for these other differential diagnoses when a patient presents with findings consistent with glaucoma:
• Vascular Differentials. Often, patients with a history of ischemic optic neuropathy in one eye can be labeled glaucoma suspects based on what may be perceived as glaucomatous cupping. The visual field defect that classically accompanies an ischemic optic neuropathy is an inferior or superior altitudinal defect, which may be incomplete and can extend into the arcades or may be located within the central 10 degrees of the macula. However, variable visual field defects are possible and the disease process can be differentiated by the lack of optic nerve cupping or notching and, instead, the presence of pallor as the manifestation of the ischemic event.
• Retinal vascular occlusions. These can also complicate the clinician’s ability to diagnose a glaucomatous optic disc. Branch retinal artery occlusions can result in visual field defects that correspond to the damaged retinal tissue and can appear arcuate-like due to having the same vascular supply. Retinal emboli or atrophic sclerotic vessels may or may not accompany a past arterial occlusion; however, the optic nerve should reveal more pallor than cupping in the affected neuro retinal rim sector.
Primary Open Angle GlaucomaPOAG is a chronic, progressive optic neuropathy with pathognomonic visual field loss and corresponding optic nerve neuroretinal rim changes. By definition, the irido-corneal angle is open on gonioscopic examination. The typical damage that ensues produces gradual excavation of the optic nerve, which can be defined by thinning, notching or cupping of the neuroretinal rim. It is not uncommon for open angle glaucoma to be asymmetric on presentation. Increased IOP is not required for the diagnosis, but it is—along with thin central corneal thickness (CCT)—a risk factor for the development of glaucoma. Making the Diagnosis The advent of spectral domain optical coherence tomography (SD-OCT) has given optometrists another means to assist in the diagnosis and management of glaucoma. Most models feature segmentation software to assess retinal layers and include a ganglion cell complex platform. Current SD-OCT systems also possess recognition software and progression or change functions to accurately track and monitor progression of RNFL loss over time. In assessing the validity of OCT test results, every clinician should understand the standardized normative database of their OCT model when evaluating OCT RNFL in glaucoma management.5 The wide variance in normal optic nerve anatomy and the different optic neuropathies mimicking glaucomatous RNFL thinning on the OCT can give the examiner a false sense of security, so it is imperative that the clinician correlate exam findings and visual field testing to help determine if nerve fiber loss on OCT is a glaucomatous process or a masquerader. The Influence of Eye Pressure The Role of Central Corneal Thickness and Corneal Hysteresis Studies have tried to link corneal hysteresis and anatomical disc changes associated with glaucomatous cupping, but these findings have shown no significant correlation.10,11 Although corneal hysteresis has yet to become a mainstream measurement in clinical practice, knowing that the cornea is a dynamic tissue that can have varying rigidity, understanding the implications of CCT and applying what is known about ethnicity and glaucoma will allow clinicians to optimally manage patients suspected of glaucoma. |
Branch retinal vein occlusions, however, would have additional retinal findings such as collateral retinal vessels bridging arteries and veins, which can cross the horizontal raphe. Residual retinal hemorrhages along the distribution of the occlusion may also be present, which helps confirm the diagnosis.
Knowing what to look for and understanding that glaucoma can arise in the presence of concurrent ocular vascular conditions ensure clinicians are prepared to monitor those patients who present with multiple comorbidities.
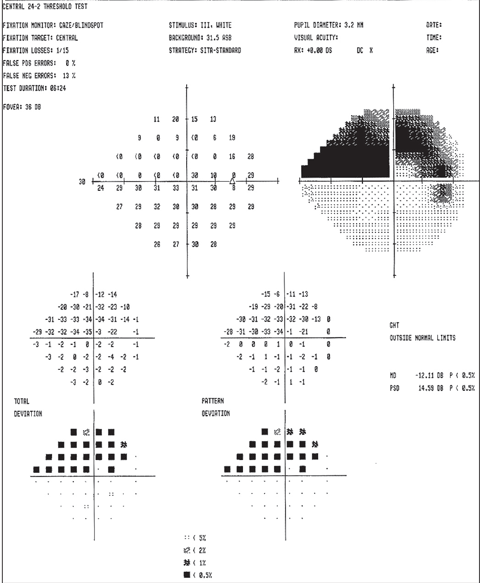
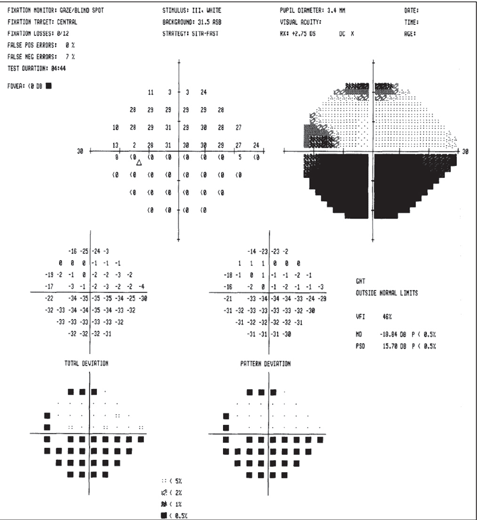
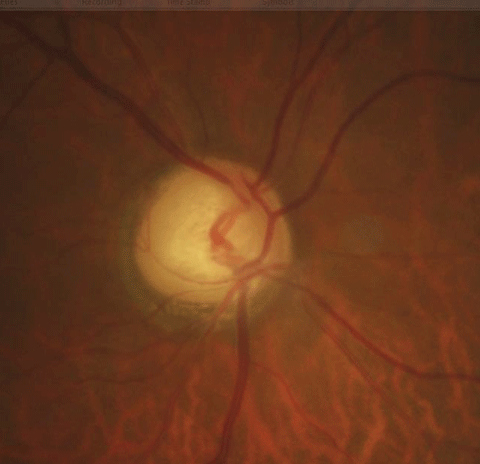 |
| Above, the fundus image shows glaucomatous thinning corresponding to the visual field. Below, the visual field shows a dense glaucomatous arcuate scotoma in the right eye. Click image below to enlarge. |
 |
• Compressive nonglaucomatous optic neuropathy. A compressive lesion can be mistaken for glaucoma, especially when the field loss and glaucomatous damage is slow and insidious. Compressive optic neuropathy can mimic glaucomatous optic nerve changes early in the disease process. The unilateral nature of the optic nerve findings and the worsening visual field defects in the presence of controlled and often normal intraocular pressure (IOP) should increase suspicion of a masquerader. A study from a tertiary referral center evaluated patients with normal tension glaucoma and found that the two most common intracranial tumors noted to cause optic nerve changes were pituitary tumors and meningiomas.12
However, several studies show that the visual field defects associated with compressive optic neuopathy most often exhibit patterns not clinically consistent with glaucomatous field loss, and the degree of optic nerve cupping can be disproportionate to the visual field defects. Researchers also discovered that, in forms of compressive optic neuropathy, nonglaucomatous optic nerve cupping and visual field loss was usually accompanied by decreased visual acuity and optic nerve head pallor.12-14
• Traumatic optic neuropathy. A history of ocular or head trauma should be considered in the differential diagnosis in any case of asymmetric optic disc cupping or suspected optic nerve pallor. Traumatic optic neuropathy can be longstanding, and patients may recall an associated event with vision loss. These patients’ visual acuity would often, but not always, be reduced, and variable visual field defects are likely. In the case of unilateral trauma, an afferent pupillary defect may be present, which would likely be accompanied by optic nerve pallor and not cupping.15 The visual field exam would also produce variable visual field defects inconsistent with the early glaucomatous visual field loss.
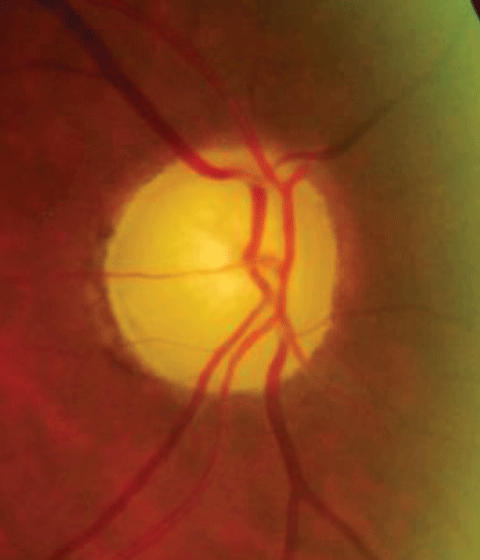 |
| The funds image shows pallor from ischemic optic neuropathy. This visual field depicts an ischemic optic neuropathy of the left eye. Click image below to enlarge. |
 |
• Optic disc swelling. Previous optic nerve swelling with resultant optic atrophy also needs to be considered when evaluating a patient who presents with findings consistent with glaucoma. An active inflammatory or swollen optic disc will intuitively lead to another diagnosis; however, optic atrophy from past events without active inflammation or disc swelling can be challenging to differentiate, even for the most seasoned clinician. Systemic diagnoses of diabetes mellitus, hypertension, anemia, a history of lymphoma or leukemia, multiple sclerosis, neuromyelitis optica, or pseudotumor cerebri can help in the differential diagnosis, as past optic nerve swelling or inflammation can change the anatomic structure and appearance of the optic nerve.
Hints It May NOT Be Glaucoma
Acuity, pupillary function, optic nerve appearance, visual field and OCT of the retinal nerve fiber layer (RNFL) and ganglion cell complex are tests that assist in the differential between glaucoma and other masqueraders. Visual acuity is generally unaffected until late in the disease process in patients with POAG. The presence or absence of an RAPD is not necessarily pathognomonic for other optic neuropathies over a glaucomatous optic neuropathy, as studies have shown 25% to 30% of POAG patients have an +RAPD.19 However, the presence of an +RAPD can sometimes be attributed to the asymmetric presentation of POAG. Therefore, the presence of an +RAPD, outside the setting of asymmetrical visual field loss, asymmetrical cup-to-disc ratio and RNFL loss could potentially suggest a nonglaucomatous disease process.20
It is essential to differentiate between optic nerve cupping and optic nerve pallor, as pallor is generally not present in early glaucomatous optic neuropathy. A more difficult task is recognizing a patient with large physiological optic nerve cupping in the presence of pallor from a nonglaucomatous optic neuropathy. Clinicians should compare the anatomy of one eye to that of the other to better evaluate any subtle anatomical changes; it is also important to take serial optic nerve head photos to assess change over time.
In a study from a tertiary referral center in which POAG patients showed progression on visual field exam, with otherwise controlled IOP, researchers noted patients with glaucomatous optic neuropathy had these common variables compared with patients with nonglaucomatous optic neuropathy: better visual acuity, history of an optic nerve hemorrhage, vertical elongation of the optic disc and visual field defects that respected the horizontal raphe of the RNFL.21 Clinicians should keep these variables in mind in cases where glaucoma patients continue to have progressive field loss in the presence of controlled IOP. Consider neuroimaging of the head and orbits to rule out other nonglaucomatous etiologies, especially in patients with normal tension and progressive visual field loss.
MeningiomasThe most common adult tumors of the optic nerve are optic nerve sheath meningiomas. They account for an estimated 2% of orbital tumors.16 Optic nerve sheath meningiomas tend to be present in younger patients, on average from 40 to 45 years old with a female preponderance, while intracranial meningiomas (which can affect the anterior and posterior visual pathway) tend to occur in patients between 45 and 55 years old.17 Orbital meningiomas can occur in close proximity to the optic nerve, within the optic nerve sheath, foramen of the optic canal, the chiasm and in the bony structure of the orbit, specifically the sphenoid wing. This can cause variable ocular signs and symptoms such as proptosis, conjunctival chemosis, decreased vision, afferent pupillary defects, color vision deficits and visual field loss. These meningiomas, in general, tend to be compressive, so depending on the location, the anterior visual pathway, optic chiasm, cavernous sinus and orbital contents can be affected. |
Visual field defects associated with glaucoma are known to respect the horizontal raphe of the retina, which is due to the anatomic distribution of the RNFL and is a reflection of how nerve fiber is lost in glaucoma. Nonglaucomatous optic neuropathies, however, can have a variety of visual field defects. Most importantly, they can cross the horizontal raphe and in some cases respect the vertical midline, which is not typical of glaucomatous field loss.22,23 Nonglaucomatous optic neuropathies often affect the papillomacular bundle, which will often give the funduscopic appearance of temporal optic nerve pallor and present as paracentral or cecocentral visual field defects early in the disease course.24
Metastatic LesionsThese lesions to the orbit always have to be considered in patients with a history of cancer, even those in remission. The most common metastases to the orbit that can cause compression are breast, lung, renal, gastrointestinal and prostate tumors.12 Not only can these lesions invade the orbit, they can also spread to the brain where they can affect the posterior visual pathway, simulating neurological or stroke-like visual field defects. These metastatic lesions can also invade the orbit and regions of the anterior visual pathway, causing compression of the optic nerve or optic chiasm and producing visual field defects, which may mimic early glaucomatous loss. |
The OCT RNFL findings of glaucoma vs. nonglaucomatous cupping can elicit overlap, making it difficult to distinguish one from the other. Glaucomatous damage noted on OCT RNFL is similar to quiescent forms of optic atrophy from past optic neuritis, ischemic optic neuropathy, optic disc drusen and loss from neurodegenerative diseases such as Parkinson’s and Alzheimer’s.25,26 Nonglaucomatous optic neuropathies will also have thinning of the RNFL; however, it is usually located temporally at the disc and should correspond to pallor of the optic nerve on exam.
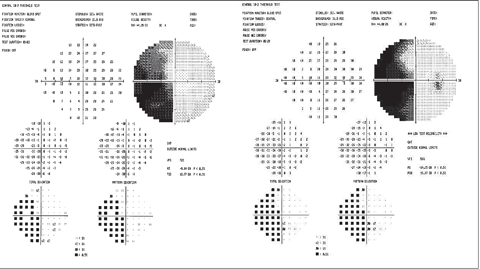 |
| Example of left hemianopsia due to stroke. Click image to enlarge. |
In conclusion, differentiating glaucoma from other nonglaucomatous masqueraders can be difficult, but the astute optometrist should be able to recognize the red flags that could alter or change the diagnosis. Understanding the glaucomatous disease process, the pathophysiology of visual loss from glaucoma and distinguishing cases that fall outside of the predicted disease course is essential. Using imaging modalities such as optic nerve photos to look for subtle optic disc neuroretinal rim and color changes, OCT retinal nerve fiber layer, ganglion cell analyses and reviewing visual field data will help to distinguish those cases of nonglaucomatous cupping. The aforementioned variables should increase awareness of the accuracy of the diagnosis so that glaucomatous masqueraders are recognized early and alternative management is instituted, avoiding potential adverse patient outcomes.
Nonglaucomatous Optic NeuropathiesClinical signs and symptoms will help differentiate between suspected glaucoma and other etiologies of optic disc changes. | |
| Glaucoma Masquerader | Key Differentiating Characteristics |
| Optic nerve pit |
|
| Ischemic optic neuropathy |
|
| Traumatic optic neuropathy |
|
| Optic atrophy (unilateral) |
|
| Foster Kennedy syndrome |
|
| Pseudo Foster Kennedy syndrome |
|
| Pituitary adenoma/apoplexy |
|
| Meningiomas of: suprasellar, cavernous sinus, optic nerve, sphenoid wing of the bony orbit |
|
| Cavernous sinus: fistula, carotid artery aneurysm |
|
| Leber’s hereditary optic neuropathy |
|
| Dominant optic atrophy |
|
| Morning glory syndrome/optic disc coloboma/tilted optic disc/optic nerve hypoplasia |
|
Typical Signs in the Differential Diagnosis of Glaucoma vs. Nonglaucomatous Optic Neuropathy | ||
| Glaucoma | Nonglaucomatous Optic Neuropathy | |
| Acuity | Normal, usually unaffected until advanced disease state | Decreased early in disease state, can lose vision at variable rates depending on etiology, often worse than 20/40 |
| Age | 50 years or older for POAG | Patients less than 50 years |
| Neuroretinal rim appearance | Inferior and superior arcuate bundle loss, vertical notching or rim thinning, generally sparing temporal rim | Pallor more often than cupping; rim can appear cupped due to loss of color |
| Presence of optic disc hemorrhage | Yes | No |
| RNFL loss by OCT imaging | Most often superior and inferior | Can be sectoral or diffuse loss, often temporal loss corresponding to the papillomacular bundle |
| Visual field defects | Most often inferior or superior arcuate/nasal step, can be paracentral or cecocentral | More often paracentral or cecocentral defects; defects crossing horizontal raphe; defects which respect vertical meridian |
| (+RAPD) | Absent in symmetrical disease states; However, can be present in 25% to 30% of cases with asymmetry of visual field and retinal nerve fiber layer thinning which coincide with neuroretinal glaucomatous damage | Can present with an +RAPD early in the disease state which does not correlate to visual field loss or nerve fiber layer/neuroretinal rim thinning |
| IOP | Can be increased, normal, or low | Often normotensive, without history of spikes or ocular hypertension |
Drs. Mazzarella and Cole are staff optometrists in the Salisbury VA Health Care System, Salisbury, NC.
|
1. National Eye Institute. Open-angle Glaucoma Defined. Accessed March 14, 2016. www.nei.nih.gov/eyedata/glaucoma. 2. Kapetanakis VV, Chan MPY, Foster PJ, et al. Global variations and time trends in prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis. Br J Ophthalmol. 2016;100:86-93. 3. Tham Y, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040. A systemic review and meta-analysis. Ophthalmol. 2014;121:2081-2090. 4. Ritch R, Shields BM, Krupin T. The Glaucomas. Vol I, II, III. St. Louis, MO: Mosby; 1996. 5. Mansoori T, Balakrishna N, Viswanath K. Influence of disc area on retinal nerve fiber layer thickness measurement by spectral domain optical coherence tomography. Indian Journal of Ophthalmology. 2014;62(5):615-8. 6. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open angle glaucoma. Arch Ophthalmol. 2002;120:701-713. 7. Deol M, Taylor DA, Radcliffe NM. Corneal hysteresis and its relevance to glaucoma. Curr Opin Ophthalmol. 2015;26:96-102. 8. Mangouritsas G, Morphis G, Mourtzoukos S, Feretis E. Association between corneal hysteresis and central corneal thickness in glaucomatous and non-glaucomatous eyes. Acta Ophthalmol. 2009;87:901-905. 9. Aghaian E, Choe JE, Lin S, Stamper RL. Central corneal thickness of Caucasians, Hispanics, Filipinos, African Americans, and Japanese in a glaucoma clinic. Ophthalmol. 2004;111:2211-9. 10. Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: A review and meta-analysis approach. Surv Ophthalmol. 2000;44:367-408. 11. Mansouri K, Leite MT, Weinreb RN, et al. Association between corneal biomechanical properties and glaucoma severity. Am J Ophthamol. 2012;153:419-27. 12. Kupersmith MJ, Krohn D. Cupping of the optic disc with compressive lesions of the anterior pathway. Ann Ophthalmol. 1984;16:948-953. 13. Kalenak JW, Kosmorsky GS, Hassenbusch SJ. Compression of the intracranial optic nerve mimicking unilateral normal pressure glaucoma. J Clin Neuro-Ophthalmol. 1992;12:230-235. 14. Jackson A, Patankar T, Laitt RD. Intracanalicular optic nerve meningioma: A serious diagnostic pitfall. Amer J Neuroradiology. 2003;24:1167-1170. 15. Chaon BC, Lee MS. Is there a treatment for traumatic optic neuropathy? Current Opinion Ophthalmol. 2015 Nov;26(6):445-9. 16. Char DH. Clinical Ocular Oncology. 2nd ed. Philadelphia: Lippincott-Raven; 1997. 17. Saeed P, et al. Optic nerve sheath meningiomas. Ophthalmology. 2003 Oct;110(10):2019-2030. 18. Lee SH, Lee EJ, Kim T. Structural characteristics of the acquired optic disc pit and the rate of progressive retinal nerve fiber layer thinning in primary open angle glaucoma. JAMA Ophthalmol. 2015 Oct;133(10):1151-8. 19. Schiefer U, Dietzsch J, Dietz K, et al. Associating the magnitude of relative afferent pupillary defect (RAPD) with visual field indices in glaucoma patients. Br J Ophthalmol. 2012;96:629-633. 20. Chew SSL, et al. Retinal nerve fiber layer loss in glaucoma patients with a relative afferent pupillary defect. Invest Ophthalmol Vis Sci. 2010 Oct;51:5049-53. 21. Greenfield DS, Siatkowski RM, Glaser JS, et al. The cupped disc. Who needs neuroimaging? Ophthalmology. 1998;105:1866-74. 22. Fraser CL, White AJ, Plant GT, Martin KR. Optic nerve cupping and the neuro-ophthalmologist. J Neuro Ophthalmol. 2013;33:377-89. 23. Greenfield, DS. Glaucomatous versus nonglaucomatous optic disc cupping: clinical differentiation. Semin Ophthalmol. 1999;14(2):95-108. 24. Pasol J. Neuro-ophthalmic disease and optical coherence tomography: glaucoma look-alikes. Curr Opin Ophthalmol. 2011;22:124-132. 25. Larrosa JM, Garcia-Martin E, Bambo MP, et al. Potential new diagnostic tool for Alzheimer’s disease using a linear discriminant function for Fourier domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55(5):3043-51. 26. Jimenez B, Ascaso FJ, Cristobal JA, Lopez del Val J. Development of a prediction formula of Parkinson disease severity by optical coherence tomography. Movement Disorders. 2014;29(1):68-74. |

