20th Annual Dry Eye ReportCheck out the other feature articles in this month's issue: |
In 2017, the Tear Film & Ocular Surface Society updated the Dry Eye Workshop (DEWS II) to reflect a decade’s worth of advances in our understanding, diagnosis, treatment and management of dry eye disease (DED). For the optometrist looking to better incorporate the findings of DEWS II into their practice, this article boils down the report’s lengthy discussion into actionable recommendations.
Start With the Right Definition
One of the major developments of the DEWS II was the Definition and Classification subcommittee’s revamping of the description of dry eye. Previously, DED was seen either as evaporative—as a result of deficient lipid layer from meibomian gland dysfunction (MGD)—or aqueous deficient (reduced tear volume).1 However, many practitioners have noted that patients can exhibit features of both subtypes. As a result, the updated definition allows for a more comprehensive representation of DED, defining it as a “multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film and accompanied by ocular symptoms in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”2
This expansive definition provides a more inclusive way to view a patient’s presenting signs and symptoms. The expanded definition creates a spectrum of DED rather than a single process, sign or symptom that affects the entire ocular surface, including the tear film, cornea, conjunctiva, eyelids and lacrimal and meibomian glands.3
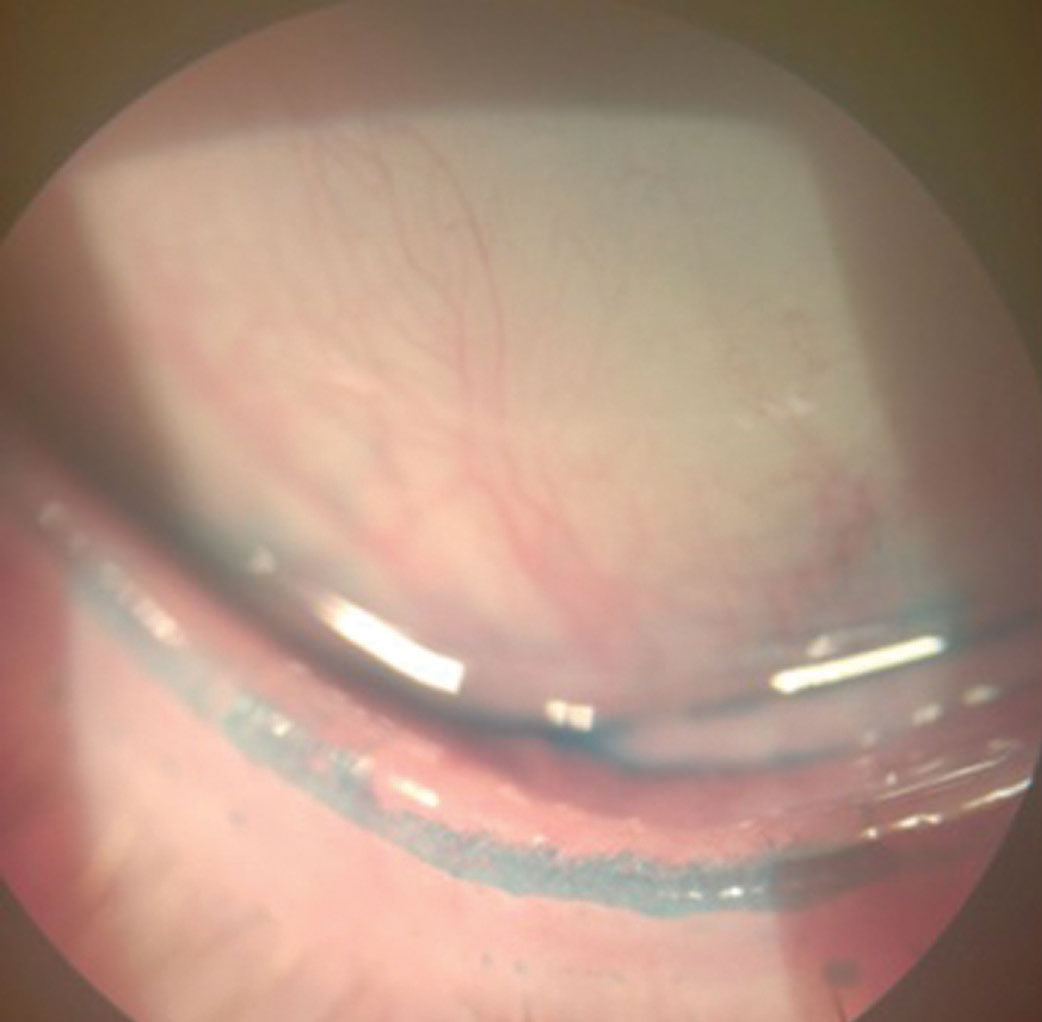 |
| Lissamine green staining of the lid margin shows >2mm of stain, which would qualify as a positive sign of lid wiper epitheliopathy, a key diagnostic criteria for dry eye disease. Click image to enlarge. |
Categorize Carefully
The DEWS II Diagnostic Methodology subcommittee’s algorithm begins with the assessment of symptoms. Patient questionnaires such as the Dry Eye Questionnaire-5 (DEQ-5) or the Ocular Surface Disease Index (OSDI) are important tools when DED is suspected, as they can often differentiate DED from other conditions that may mimic its symptoms.1
It’s All About Balance
The new understanding of DED as a disruption of homeostasis on a spectrum of ocular surface dysfunction means identifying the factors contributing to a patient’s profile is key. While there may be a predominant cause, concurrent contributing factors are also possible.
DEWS II recommends using three tests and techniques to identify and subtype DED. These are minimally invasive, clinically applicable and maintain high objectivity.1 Clinicians should test for these key homeostasis markers in symptomatic patients using a positive screening questionnaire such as DEQ-5 (with a score >6) or OSDI (with a score >13). Testing should be performed in order from least to most invasive to minimize a test’s effect on subsequent results:
 |
| Want to read more about the DEWS II reports and peruse the diagnostic and treatment algorithms? Check out our coverage of each subcommittee report: DEWS II: Redefining Dry Eye - August 2017 |
Noninvasive tear break-up time (TBUT). This is always recommended over traditional fluorescein TBUT, as fluorescein reduces tear film stability and impacts the accuracy of the measurement. Noninvasive TBUT can be measured with devices such as a corneal topographer.1 However, if fluorescein is used, it is recommended that the test strip be dry and applied to the outer canthus to decrease any irritation of the ocular surface with measurements read one to three minutes after instillation.1 A positive test result is tear break-up less than 10 seconds after blink.
Tear hyperosmolarity. This has the highest correlation to DED severity of available clinical tests, and tear osmolarity is the single best metric to diagnose and classify DED, according to the DEWS II diagnostic and methodology report.1 A positive result is any reading greater than 308mOsm/L or a difference of more than 8mOsm/L between eyes.
Ocular surface staining. Conjunctival and lid margin damage is best viewed with lissamine green staining, and corneal damage is best viewed with fluorescein dye.1 Considered a late-stage sign of DED, staining in either eye is a positive test result. Ocular surface staining is defined as more than five corneal spots, greater than nine conjunctival spots or lid margin staining (lid wiper epitheliopathy) greater than 2mm in length.
While only one abnormal test result is required to make a diagnosis, if a clinician only has access to a limited number of homeostasis markers, all of which come back negative, the clinician should refer the patient to rule out other markers of homeostasis before excluding the diagnosis of DED.1
Once DED is confirmed, further testing such as meibography, lipid interfermometry and tear volume measurement can further subtype and determine where on the DED continuum the patient falls, as well as determine the severity and help guide treatment.3
A Staged Treatment Approach
Because DED exists on a spectrum, the management and treatment requires a similar approach. DED can require several levels of care simultaneously. The DEWS II management and therapy algorithm is not a rigid sequential approach, but a guide to aid in the treatment.4 In some cases, the initial therapy may be continued in addition to any new therapies in subsequent stages.4
Stage one. Patient education, as with any condition, is an important first step and helps to promote treatment compliance and guide patient expectations, especially in refractory cases. Additionally, patients with signs of dry eye without symptoms should be counseled on their condition along with potential worsening prior to any ocular surgery or when considering contact lens wear.
Patients can start by modifying their local environment by adding humidifiers in particularly dry conditions, as well as modifying other environmental issues such as prolonged digital device use and contact lens wear.4 Dietary modifications may help as well, as evidence shows that diet and nutritional supplementation play a role in managing DED. Increasing water intake is a simple recommendation with significant benefits for patients with DED.4 At the time of the DEWS II report, the use of omega-3 supplementation for dry eye was still unclear, with conflicting reports of efficacy.4 Since DEWS II, the DREAM study found that fish oil did not perform significantly better than its olive oil placebo in treating dry eye.5
 |
| DEWS II staged DED recommendations. Adapted from: Jones L, Downie LE, Korb D, et al. Ocul Surf. 2017;15(3):575-628. Click image to enlarge. |
Clinicians should identify topical or systemic medications that may contribute to DED such as antihypertensives, antihistamines and anti-anxiety medication. Once identified, patients can consider dose adjustments, switching to another medication, discontinuing the medication or more aggressive management of the induced dry eye.4 When the offending topical agent is preserved with benzalkonium chloride, clinicians should recommend switching to a different preservative such as Polyquad or a preservative-free solution to help minimize damage to the ocular surface, which may occur in those who require frequent dosing.4
Artificial tears are a mainstay of DED therapy and are used as palliative therapy; however, these over-the-counter products do not work to address the pathophysiology of DED.4 Artificial tears vary in osmolarity, viscosity and pH—all of which impact their efficacy for individual patients. Higher viscosity agents such as carboxymethylcellulose, hyaluronic acid, HP-guar, polyvinyl alcohol and propylene glycol are typically recommended for overnight use.4 In more advanced cases, higher viscosity agents are used more frequently to help prevent ocular surface desiccation. Lipid-containing drops are formulated with various oils such as mineral oil and phospholipids to help restore the tear film lipid layer, and are beneficial in cases of evaporative dry eye.4
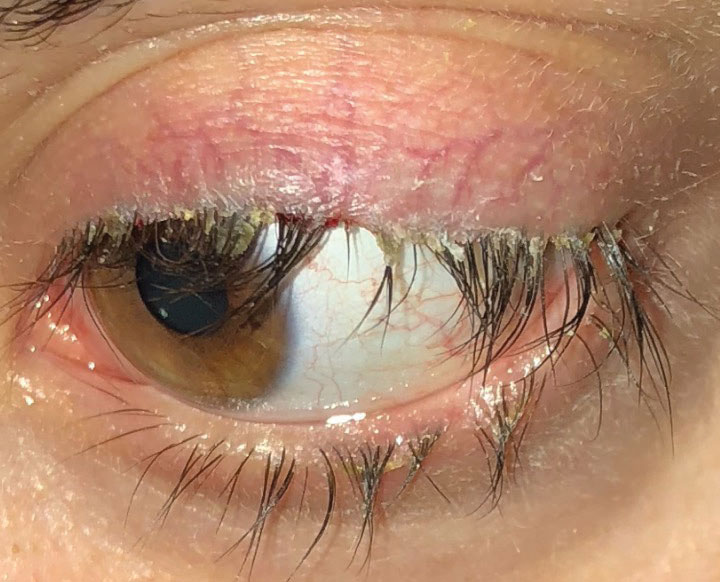 |
| This patient with anterior blepharitis required stage two treatment, which can include topical antibiotics or antibiotic-steroid combination medications, along with lid hygiene and warm compresses. Click image to enlarge. |
Lid hygiene is important in managing any conditions that may further contribute to DED, such as blepharitis, MGD and ocular rosacea. Although lid scrubs using a mild dilution of baby shampoo have long been a common recommendation, studies show commercially available eyelid cleaners provide reduced ocular surface MMP-9 levels, improved lipid layer quality and are overall better tolerated compared with baby shampoo.4 Research also suggests baby shampoo may have an adverse effect on goblet cell function, and the DEWS II recommends commercially available lid cleansing products over the use of baby shampoo.4
Warm compresses are a proven at-home treatment for MGD; however, compliance is typically poor due to the time required and the difficulty of maintaining the appropriate temperature of no more than 45°C for an extended period of time.4 Patients can keep the cloth warm for longer by wrapping several cloths around each other in a bundle format. Patients should use a warm wet compress for at least five minutes.4 Many commercially available devices can maintain the therapeutic temperature for longer periods of time; the Bruder mask (Bruder Healthcare), for example, maintains heat levels for 10 to 15 minutes.6
Stage two. Patients unresponsive to stage one therapies should initiate stage two treatment options. Switch to non-preserved artificial tears if corneal toxicity is a concern and recommend overnight treatments such as increased viscosity tears and ointments. Moisture chamber spectacles to slow tear evaporation and minimize airflow over the ocular surface may be beneficial.
If tear volume is a concern, punctual occlusion with collagen, silicone or surgical intervention in more advanced cases can help with tear conservation. Punctal occlusion is most successful in combination with other DED treatments.4
Stage two dry eye often responds to topical pharmaceutical agents such as cyclosporine A, which is an immunomodulatory drug with anti-inflammatory properties that inhibit the IL-2 activation of lymphocytes.4 Topical cyclosporine, FDA-approved for moderate to severe DED, reduces markers of inflammation, decreases tear osmolarity, improves conjunctival goblet cell density and improves tear production measured via Schrimer’s.4 In 2018, Cequa (Sun Pharmaceuticals) gained FDA approval and provides a higher concentration of cyclosporine at 0.9% compared with the 0.05% of Restasis (Allergan).7
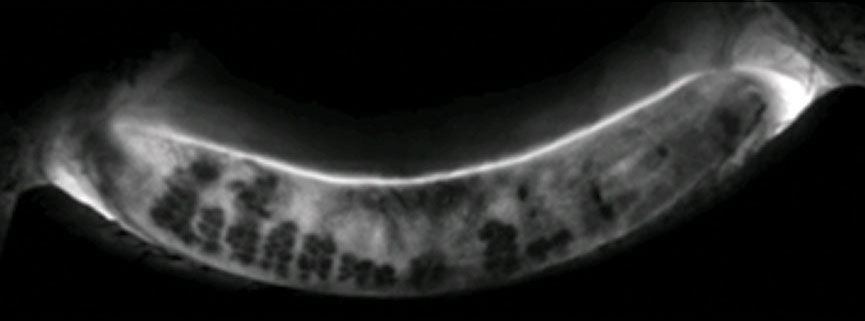 |
| This Lipiscan image of a patient’s meibomian glands shows extensive areas of gland loss and truncation consistent with the patient’s complaints of dry eye. Click image to enlarge. |
Xiidra (lifitegrast, Novartis) is a small molecule integrin antagonist that binds to the cell surface protein found on leukocytes and blocks the integrin lymphocyte function associated antigen-1 and cognate ligand intercellular adhesion molecule-1 interactions.8-10 In vitro studies show Xiidra may inhibit the recruitment of previously activated T-cells, the activation of newly recruited T-cells and the release of pro-inflammatory cytokines—interrupting the perpetual cycle of inflammation that promotes DED.8-10
Clinicians can also consider using low-dose topical steroids such as Lotemax (loteprednol etabonate 0.5%, Bausch + Lomb) as a pretreatment or concomitantly with cyclosporine or lifitegrast in the early phases of treatment and tapered after a few weeks. A study found non-preserved steroids, such as 0.01% dexamethasone, can improve patient symptoms and findings of chronic ocular surface irritation that was previously unresponsive to various preserved topical steroids such as 0.2% loteprednol, 0.1% fluorometholone and 1% prednisolone.4
Stage two patients often present with lid involvement such as anterior blepharitis. A short course of topical antibiotics or an antibiotic/steroid combination ointment applied directly to the lid margin may help, in combination with lid hygiene and warm compresses.
Treatment of Demodex, if present (often with refractory blepharitis), will help alleviate patient symptoms and decrease any contributing impact on DED.4 The use of tea tree oil, which exhibits antimicrobial, anti-inflammatory, antifungal and antiviral properties that are toxic to Demodex mites, has grown in popularity for its effectiveness in eradicating Demodex mites from the hair follicles at the lash margin.4 One study shows weekly lid scrubs with 50% tea tree oil combined with daily lid hygiene with tea tree shampoo is an effective treatment for Demodex.4,11
Tea tree oil can be toxic to the ocular surface and can cause stinging and irritation if used in its pure form.4 Pre-formulated wipes are now commercially available at 25% concentration and reduce the risk of toxicity to the ocular surface compared with stronger concentrations.4
Oral tetracyclines, such as doxycycline, can treat ocular rosacea, MGD and blepharitis that contribute to DED. These broad-spectrum antibiotics with anti-inflammatory properties can decrease several inflammatory mediators such as collagenase, phospholipase A2 and several MMPs.4 No consensus exists on the optimal dosage of oral doxycycline for treating DED due to MGD, and regimens range from 20mg to 200mg daily in monthly intervals.4 The lowest dose is always preferably to help minimize possible side effects of photosensitivity and gastrointestinal upset.
Oral macrolides, and azithromycin specifically, can be as effective as doxycycline in treating MGD.4 Azithromycin works by inhibiting pro-inflammatory cytokines and is potent against gram-negative microorganisms associated with posterior blepharitis.12 While the dosing of azithromycin for MGD remains controversial, clinicians should use a shorter course of treatment—500mg on first day and 250mg for four days after for a total of five days—to minimize gastrointestinal upset and boost cost efficiency.
MGD can be treated in office with instruments such as LipiFlow (Johnson & Johnson Vision) or intense pulsed light (IPL). LipiFlow is designed to simultaneously heat glands to therapeutic levels of 42.5°C and evacuate the gland contents, which can significantly improve patient symptoms, meibomian gland secretion and TBUT with one study showing efficacy of one treatment up to three years.4 IPL therapy, which uses a handheld device to deliver pulses of non-coherent light between 500nm and 1200nm, can improve meibomian gland function and dry eye symptoms when used with manual meibomian gland expression. A retrospective multicenter review found that IPL therapy was a safe and effective treatment for evaporative dry eye.4
Stage three. These patients require more advanced dry eye therapies such as oral secretagogues. Pilocarpine and cevimeline, both cholinergic agonists, are commercially available for oral administration in the treatment of Sjögren’s syndrome (SS)-associated DED.4 One study found SS patients treated with oral pilocarpine had improvement of symptoms and ocular surface staining with rose bengal, goblet cell density and TBUT; however, it did not improve tear production via Schrimer’s.4 Cevimeline showed a better side effect profile compared with pilocarpine, and patients had significant improvements in subjective assessment of ocular dryness, dry mouth and increased salivary and lacrimal flow rates.4
In more advanced cases of DED where topical pharmaceutical agents do not provide adequate relief (often in patients with underlying systemic conditions such as SS), autologous serum can be beneficial. Autologous serum is derived from the patient’s own blood and contains many biochemical characteristics similar to that of human tears, such as pH, nutrients, vitamins, albumin, fibronectin and epithelial and nerve growth factors.4 Research shows autologous serum and other blood-derived tear substitutes increase corneal epithelial wound healing, inhibit the release of inflammatory cytokine and increase the number of goblet cells and mucin expression.4
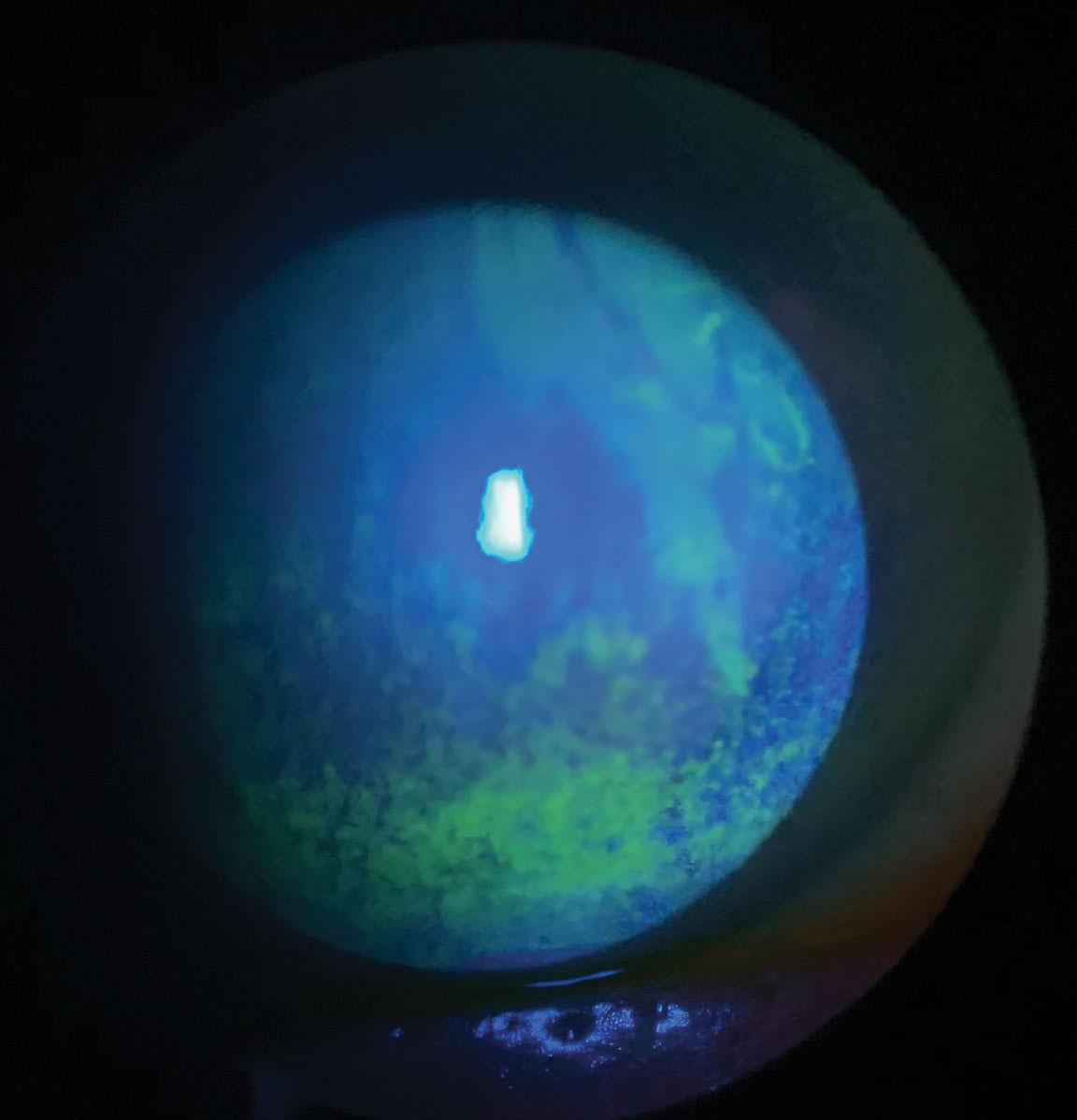 |
| This patient with dry eye has extensive punctate epithelial keratitis and decreased tear break-up time. Treatment requires consideration of patient symptoms, underlying contributory systemic conditions and medication use. Click image to enlarge. |
Autologous serum can be formulated at specialized compounding pharmacies at various concentrations, depending on severity of symptoms.4 The formulations are non-preserved and can be kept frozen at -20°C for up to nine months, but are only good for about 24 hours after thaw.4 One study found treatment with autologous serum improved patient symptoms as early as 10 days in 60% of patients and two months in 79%.4 While patient symptoms, TBUT and corneal staining improved, Schirmer’s scores remained the same and ocular surface disease recurred after discontinuation of treatment.4
Allogenic serum, which works similarly to autologous serum, is derived from the blood of a relative or individual of similar blood typing.
Bandage contact lenses can help maintain ocular comfort and corneal integrity and prolong ocular surface moisturization.4 Soft contact lenses are typically used on an extended wear basis, although these patients should be advised of increased risk of infection and monitored regularly for complications. A recent study found silicone hydrogel lenses used as a bandage lens in SS patients provided significant improvement in visual acuity for up to six weeks after discontinuing wear, as well as improvement in OSDI scores, TBUT and corneal staining.4
Gas permeable scleral lenses are an option for moderate to severe DED and can provide a repository of tears between the lens and the ocular surface.4 However, once a centralization of neuropathic pain occurs, the use of any bandage lens may be insufficient for reducing symptoms.4
Stage four. Treatment at this stage is for refractory cases of DED. Chronic use of low-dose topical corticosteroids may be warranted to help control persistent ocular surface inflammation. Careful monitoring of secondary cataract formation or increased intraocular pressure is necessary for long courses of treatment.
Severe DED with persistent epithelial defects or corneal ulceration and scarring may be successfully treated by amniotic membrane grafts. These consist of cryopreserved human amniotic membrane, which contains a wide variety of neuropeptides and neurotransmitters.4 These devices are inserted similarly to a scleral lens and typically dissolve in one week. One study showed symptom improvement for four months in dry eye patients treated with a Prokera Slim (Bio-Tissue) for five days.4
In the more severe cases of DED, surgical intervention may be necessary. Tarsorrhaphy is a temporary or permanent surgical procedure to partially or totally close the eyelids to decrease ocular surface exposure and tear evaporation in patients where all other treatments have failed. Removal of excessive conjunctivochalasis can help patients whose redundant conjunctival tissue exacerbates their dry eye symptoms as well as complaints of epiphora.4
The management options for dry eye have exploded over the past decade, and the DEWS II report provides the eye care community a much-needed road map to best identify patients who would benefit from treatment and tailor management to their individual needs.
Dr. Tolud practices at South Jersey Eye Physicians and specializes in ocular disease management.
|
1. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539-74. 2. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-83. 3. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II Report executive summary. Ocul Surf. 2017;15(3):802-12. 4. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628. 5. Dry Eye Assessment and Management Study Research Group, Asbell PA, Maguire MG, et al. n-3 fatty acid supplementation for the treatment of dry eye disease. N Engl J Med. 2018;378(18):1681-90. 6. Insight Optometry. Bruder Eye Hydrating Mask. https://insightoptometry.com/blog/bruder-moist-heat-eye-compress. Accessed March 19, 2019. 7. Sun Pharma Announces U.S. FDA Approval of CEQUA to Treat Dry Eye Disease. Sun Pharmaceutical Industries. https://cequapro.com/pdf/cequa-news-release.pdf. August 16, 2018. Accessed March 7, 2019. 8. Murphy CJ, Bentley E, Miller PE, et al. The pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogs. Invest Ophthalmol Vis Sci. 2011;52:3174-80. 9. Sun Y, Zhang R, Gadek TR, et al. Corneal inflammation is inhibited by the LFA-1 antagonist, lifitegrast (SAR 1118). J Ocular Pharmacol. 2013;29:395-402. 10. Zhong M, Gadek TR, Bui M, et al. Discovery and development of potent LFA-1/ICAM-1 antagonist SAR 1118 as an ophthalmic solution for treating dry eye. ACS Medicinal Chemistry Letters. 2012;3:203-6. 11. Gao Y, Di Pascuale MA, Li W, et al. In vitro and in vivo killing of ocular Demodex by tea tree oil. Br J Ophthalmol. 2005;89:1468-73. 12. Kashkouli MB, Fazel AJ, Kiavash V, et al. Oral azithromycin versus doxycycline in meibomian gland dysfunction: a randomised double-masked open-label clinical trial. Br J Ophthalmol. 2015;99(2):199-204. |

