20th Annual Dry Eye ReportCheck out the other feature articles in this month's issue: |
Nearly all eye care providers will treat dry eye and, while we have many arrows in our dry eye treatment quiver, one, cyclosporine, is of particular interest. This agent first gained prominence in the United States when Restasis (cyclosporine A ophthalmic emulsion 0.05%, Allergan) launched in 2003. Since then, a number of similar pharmaceutical options have entered the domestic and foreign markets, with even more new formulations in clinical trials. With this expansion, optometrists should understand how the array of options differ, how they’re similar and other details of their mechanisms to pair them with the appropriate patients. This article reviews what the academic literature and published clinical trials show regarding the various cyclosporines around the globe.
Inflammation's Role
Dry eye—a multifactorial disease of the tears and ocular surface that causes discomfort, visual disturbance and tear film instability—is estimated to affect more than 40 million Americans, and that number is anticipated to increase drastically.1-4 Inflammation is a major factor of dry eye and it is primarily mediated by CD4+ T cells.5
The initial induction of inflammation varies and can include one of two systemic autoimmune diseases. One is Sjögren’s syndrome (SS), which results in lymphocytic infiltration of the lacrimal gland, leading to aqueous-deficient dry eye. The other is meibomian gland dysfunction (MGD), which reduces the lipid component of the tear film leading to evaporative dry eye.4,6 Irrespective of any identifiable underlying local or systemic inflammatory disorder, dry eye seems to be invariably associated with chronic inflammation of the ocular surface, although it is not known whether the local inflammation is causative or simply occurs as a consequence of ocular dryness. Regardless, recognition of the role of inflammation in dry eye has been a crucial factor in facilitating dry eye treatment.
Since inflammation plays such a significant role in dry eye, promoting ocular surface disruption and symptoms of irritation, a number of anti-inflammatory treatments are currently available for its management. Many more anti-inflammatory medications are in the development or clinical trials phases. These agents inhibit the expression of inflammatory mediators on the ocular surface, thereby restoring the secretion of a healthy tear film and reducing the signs and symptoms of afflicted patients.
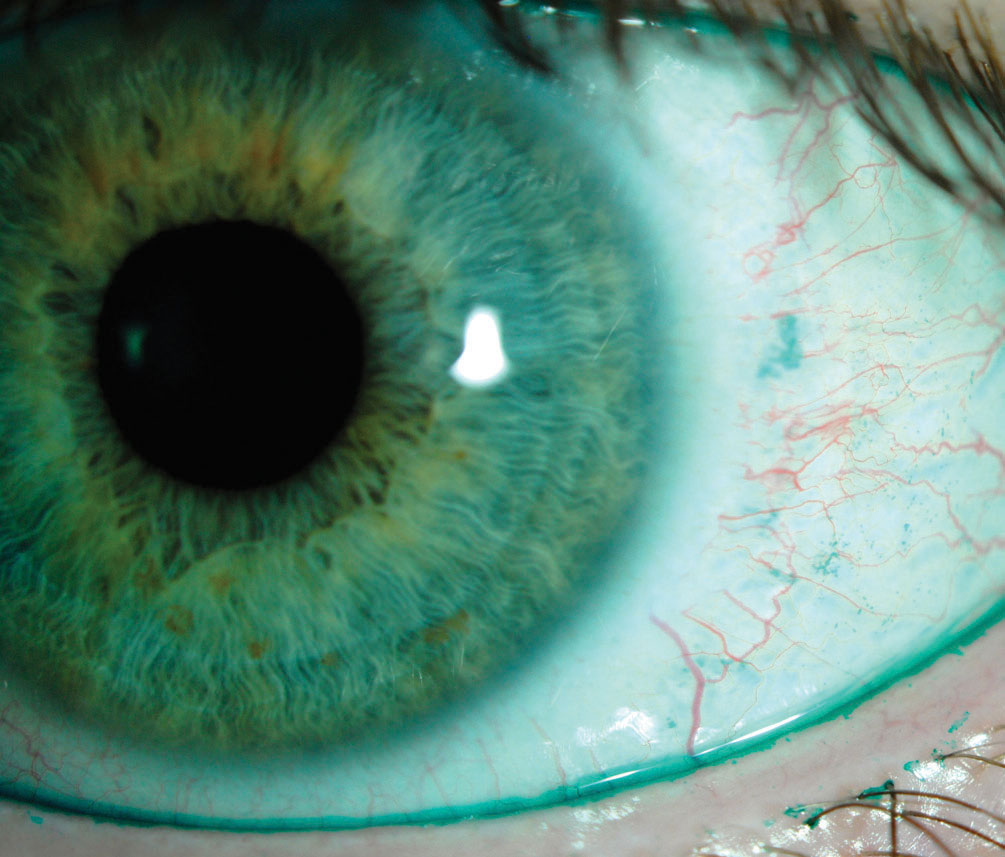 |
| This positive lissamine green conjunctival staining indicates dry eye, whereas the lid margin staining is indicative of lid wiper epitheliopathy. Cyclosporine drugs offer these kinds of patients relief. Photo by Jalaiah Varikooty. Click image to enlarge. |
CsA Overview
Cyclosporine A (CsA) has principal pharmacologic action of suppressing the activation and function of T-lymphocytes, acting as an immunosuppressant and inhibitor of cell death. Patients with severe dry eye often have a poor response to the standard twice-daily dosing of Restasis, even after several months of treatment, and often benefit from an increased dosing application of three or four times per day.8 Since frequent dosing can be a hassle for patients, researchers are seeking better drug delivery systems that can increase the concentration of CsA in the cornea and conjunctiva. While CsA drops may be compounded for patients at higher concentrations, the access for patients to obtain them from a compounding pharmacy may be limited and cost-prohibitive. But new options include the recently FDA-approval of Cequa (CsA 0.09%, Sun Pharmaceuticals), the compounded drop Klarity-C (Imprimis) and formulations not yet available in the US, including Ikervis (CsA 0.1%, Santen), Modusik-A Ofteno, TJ Cyporin and Papilock Mini.
CsA Mechanism of Action
Research shows commercially available topical cyclosporine 0.05%, such as Restasis, or 1% compounded preparations are effective in several inflammatory conditions including vernal conjunctivitis, Thygeson’s superficial punctate keratitis, non-infectious keratitis and MGD.17 The American Academy of Ophthalmology now considers CsA a dry eye treatment option in its Preferred Practice Pattern.18 The immunomodulating effects of cyclosporine A are achieved through binding with cyclophilins, which are a group of proteins. Cyclophilin A, which is found in the cytosol, and the cyclosporin-cyclophilin A complex inhibits a calcium/calmodulin-dependent phosphatase, calcineurin, which is thought to halt the production of the transcription of T-cell activation by inhibiting IL-2.19 Cyclophilin D is located in the matrix of mitochondria. Cyclosporine A-cyclophilin D complex modulates the mitochondrial permeability transition pore thereby inducing a mitochondrial dysfunction and cell death.20 The reduction in inflammation, via inhibition of T-cell activation and down-regulation of inflammatory cytokines in the conjunctiva and lacrimal gland is thus thought to allow enhanced tear production.21-25 Topical cyclosporine also increases goblet cell density and decreases epithelial cell apoptosis.26
Treatment Protocol
When starting CsA treatment for a patient, we commonly educate them on the somewhat extended and variable duration of using the eye drop before it may result in improvement of their symptoms. Relief may take three weeks to three months after initiating CsA.27
In a survey of 144 patients in an extension study of the initial clinical trial of CsA (both the 0.05% and 0.1% formulation), 62.5% reported that their dry eye symptoms began to resolve after three months of treatment.27 Patient reports of onset of symptom relief was faster in two larger survey studies involving more than 8,000 dry eye patients in which more than half of patients reported CsA was effective within three to five weeks.28,29 It is unclear if the reduction in symptoms and staining (sodium fluorescein and rose bengal) was secondary to active CsA vs. lubrication from CsA vehicle.28,29 In a prospective study of 158 patients treated with CsA, 22% reported no change in their symptoms as measured by the ocular surface disease index (OSDI) over an average of eight to 10 months of follow-up.30 That large range in time to improvement may be because severe DED is so severe that patients notice slight improvements faster than someone who has more moderate symptoms.
Patient education regarding side effects of CsA is also an important conversation to improve compliance. The most commonly reported side effect of CsA 0.5% is ocular burning (reported in 17% of patients), which is also the most cited reason for discontinuation of CsA.23,30-32 Topical steroid use prior to instillation of CsA may help reduce this burning sensation.33 Pretreatment with topical Lotemax (loteprednol etabonate 0.5%, Bausch + Lomb) induction two weeks before the initiation of topical CsA 0.05% can provide more rapid relief of dry eye signs and symptoms and greater efficacy than CsA and artificial tears alone.33 No studies currently demonstrate findings that would suggest systemic absorption of topical CsA for ophthalmic use.
Pathophysiology ReviewEvidence from the past decade shows dry eye-related ocular surface inflammation is mediated by lymphocytes.9 Based on earlier immunohistopathological evaluations, patients with both SS-related as well as non-SS dry eye have identical conjunctival inflammation manifested by T-cell infiltrates and upregulation of CD3, CD4 and CD8 as well as lymphocyte activation markers CD11a and HLA-DR.10 These results suggest symptoms of dry eye may be dependent on T-cell activation and resultant autoimmune inflammation. Multiple other studies followed and demonstrated the role of pro-inflammatory cytokines and matrix metalloproteinases (MMPs) in the pathogenesis of dry eye disease. Interleukin (IL)-1 is one of the most widely studied cytokines accompanying dry eye. An increase in the proinflammatory forms of IL-1 (IL-1α and mature IL-1b) and a decrease in the biologically inactive precursor IL-1b have been found in the tear film of dry eye patients.11 The source of the increased levels of IL-1 was thought to be the conjunctival epithelium based on immunohistochemical studies.11 More recently, reactive nitrogen species expressed by conjunctival epithelial cells have been recognized as playing a role in the pathogenesis or self-propagation of SS-related dry eye.12 In the same study, IL-1b, IL-6, IL-8, and tumor necrosis factor (TNF)-α also play a significant role in SS-related dry eye compared with normal eyes. The response of cells to extracellular stimuli, such as ocular surface stress (including changes in the composition of the tear film or hyperosmolarity and ultraviolet light exposure) is mediated in part by a number of intracellular kinase and phosphatase enzymes.13 Mitogen-activated protein (MAP) kinases are integral components of parallel MAP kinase cascades activated in response to a number of cellular stress, including inflammatory cytokines (e.g., IL-1 and TNF-α), heat shock, bacterial endotoxin and ischemia. Activation of these MAP kinase homologues mediates the transduction of extracellular signals to the nucleus and is pivotal in regulation of the transcription events that determine functional outcome in response to such stresses. Researchers have identified these stress-activated protein kinases in the tear film of patients with dry eye. Activation of these stress pathways results in transcription of stress-related genes, including MMPs, mainly MMP-9.14 In another study, MAP kinases were found to stimulate the production of inflammatory cytokines including IL-1, TNF-α and MMP-9 and thereby causing ocular surface damage.15 Hyperosmolarity induces inflammation in human limbal epithelial cells by increasing expression and production of pro-inflammatory cytokines and chemokines such as IL-1b, TNF-α and IL-8.16 This process appears to be mediated through activation of the C-Jun N-terminal kinases and MAPK signalling pathways. The science of dry eye pathogenesis may be complex, but it gives researchers numerous targets for potential therapies. All of these inflammatory mediators and pathways should be considered important, and should also be kept in mind when discussing treatment strategies. |
New CsA Options
The option of cyclosporine therapy is evolving from a single agent to a burgeoning category of choices. As Restasis is quite familiar to practicing optometrists, we will concentrate on the newer agents.
Cequa (Sun Pharmaceutical)—preclinically referred to as OTX-101—is a nanomicellular topical 0.09% formulation recently FDA approved for DED treatment. This aqueous-based solution (as opposed to the oil-based emulsion of Restasis), aims to deliver therapeutic concentrations of CsA with minimal discomfort.
Investigators believe oil-based preparations are poorly tolerated by patients and lead to low bioavailability due to higher attraction of CsA to the lipophilic vehicle in contrast to the highly hydrophilic tissue.34 Therapeutic levels of emulsion forms are reached in the tissues only after a large number of instillations, raising concerns for patient compliance. CsA ocular emulsions have increased CsA tissue levels; however, manufacturing problems are associated with high cost and potential toxicity over long-term use.35
The non-ionic surfactant polymers included in the Cequa formulation are FDA approved. Such safety from these polymers can be justified by the lack of toxicity from the preliminary results performed in human-derived corneal and retinal cells.36 In addition, the negligible charge of the formulation helps prevent repulsion of the formulation with negatively charged cell surfaces, improving its interaction with the ocular cells.37
A comparative pharmacokinetics study between cyclosporine concentrations after single topical admin-istration of Restasis 0.05% and of Cequa 0.05%—another CsA nanomicellar formulation—shows 3.84-fold higher CsA concentrations in the tears for Restasis than Cequa.34 This is likely due to interaction of the oil-based vehicle with the outer oily layer of the tear. Similarly, the superior eyelid, which is primarily composed of a thin skin, absorbed significant CsA concentrations (1.98-fold) from oil-based Restasis in contrast to Cequa.34 Systemic CsA exposure was also substantially lower for both of the formulations in comparison with ocular tissues, indicating lower chances of systemic side effects. The favorable pharmacokinetic in vivo results obtained for Cequa led to its advancement into clinical trials.
The majority of the ocular adverse events were mild and transient only at the instillation site with Cequa treatment groups, but no serious ocular adverse effects were reported in the Phase II/III study. Cequa 0.05% and 0.09% treatment groups demonstrated an equivalent safety and tolerability profiles.
Cequa is also the first DED product candidate that has demonstrated significant improvement for both conjunctival staining and anaesthetized Schirmer’s test, apart from reduction in corneal staining.38 The results of a Phase III study demonstrate that Cequa 0.09% significantly improved sign- and symptom- end points of DED.39 The trial also proved the ocular safety of Cequa is consistent with the previous studies.39
Cyclasol (Novaliq) is another preservative-free clear solution the treatment of moderate-to-severe DED. Using the company’s EyeSol technology based on semifluorinated alkanes, the new formulation does not use water, oils, surfactants or preservatives. An ex vivo model supports the higher bioavailability potential whereby, after initial application, Cyclasol 0.05% passes through the corneal barrier in as little as two and a half hours; after 8.5 hours, Restasis had not penetrated.40
Researchers believe Cyclasol has a significantly greater local bioavailability based on penetration into the cornea, compared with Restasis and Ikervis (eightfold and twofold respectively).
Efficacy, safety and tolerability of a 0.1% formulation of Cyclasol were evaluated in a Phase II, multicenter, randomized, vehicle-controlled clinical trial, double-masked between Cyclasol (0.5% and 0.1%) and vehicle with open-label comparator (Restasis) at twice daily for 16 weeks.42 Cyclasol showed a consistent reduction in corneal and conjunctival staining compared with both vehicle and Restasis over the 16-week treatment period with an early onset of effect (at day 14).
A mixed-effect-model approach demonstrated that the Cyclasol drug effect was statistically significant over vehicle.42 This analysis suggests a significant Cyclasol effect for OSDI as a symptom parameter. The number of ocular adverse events were low in all treatment groups. No clear differences between the two Cyclasol concentrations were observed in signs, symptoms or safety parameters analyzed for the clinical data, and this finding was supported by the modeling data.
Ikervis (Santen) is a preservative-free formulation being evaluated, yet unavailable, in the United States.
In a clinical trial, 246 DED patients with severe corneal fluorescein staining were randomised to one drop of Ikervis or vehicle daily at bedtime for six months.43 The primary endpoint was the proportion of patients achieving by month six at least a two-grade improvement in corneal staining and a 30% improvement in symptoms, measured with the OSDI.43 The proportion of responders in the Ikervis group was not statistically significant (28.6%, compared with 23.1% in the vehicle group).43
The severity of corneal staining improved significantly from baseline at six months with Ikervis compared with the vehicle. The proportion of Ikervis-treated patients with a three-grade improvement in corneal staining at six months (from four to one) was 28.8% compared with 9.6% of vehicle-treated subjects, but this was a post hoc analysis. The mean change from baseline in the 100-point OSDI score was -13.6 with Ikervis and 14.1 with vehicle at month six.43 In addition, no improvement was observed for Ikervis compared with vehicle at month six for other secondary endpoints, including ocular discomfort score, Schirmer test, use of concomitant artificial tears, investigator’s global evaluation of efficacy, tear break-up time, lissamine green staining, quality of life score and tear osmolarity.43
In a different six-month trial, 492 DED patients with moderate-to-severe corneal staining were also randomized to Ikervis or vehicle daily at bedtime for six months.44 The two primary endpoints were the change in corneal staining score, and the change in global score of ocular discomfort unrelated to study medication instillation, both measured at six months.44 Researchers noted a small but statistically significant difference in corneal staining between the treatment groups at six months in favor of Ikervis.44
Klarity—C (ImprimisRx) is yet another formulation to be used in the treatment of signs and symptoms of dry eye.45 It contains 0.1% cyclosporine in a chondroitin sulfate ophthalmic emulsion, which is said to have short-term anti-inflammatory properties.46 The product is supplied in a multi-dose bottle for convenience and potential cost savings. In a three-month study of 75 patients, OSDI scores improved in all patients, and 56% had at least a 20 -point change in OSDI.45 In addition, 81.2% of eyes had a reduction in corneal staining after three months of topical therapy.45
International medications. A few other cyclosporine formulations, unavailable in the US, have been mentioned in the literature; however, minimal research has been published to support efficacy and safety. Papilock Mini was approved in 2008 in Japan to treat vernal keratoconjunctivitis (VKC) at a dosage of three times daily. Lacrinmune (topical cyclosporine 0.05% emulsion, Bausch + Lomb) launched in Argentina and is dosed twice daily for the treatment of keratoconjunctivitis sicca (KCS). TJ Cyporin (cyclosporine 0.05% solution, Taejoon Pharmaceuticals) is available in some countries in Asia. Modusik-A Ofteno (cyclosporine 1%, Labratorios Sophia) was approved in 2003 to treat KCS and is available in Mexico, Chile, Columbia, Peru and Equador.
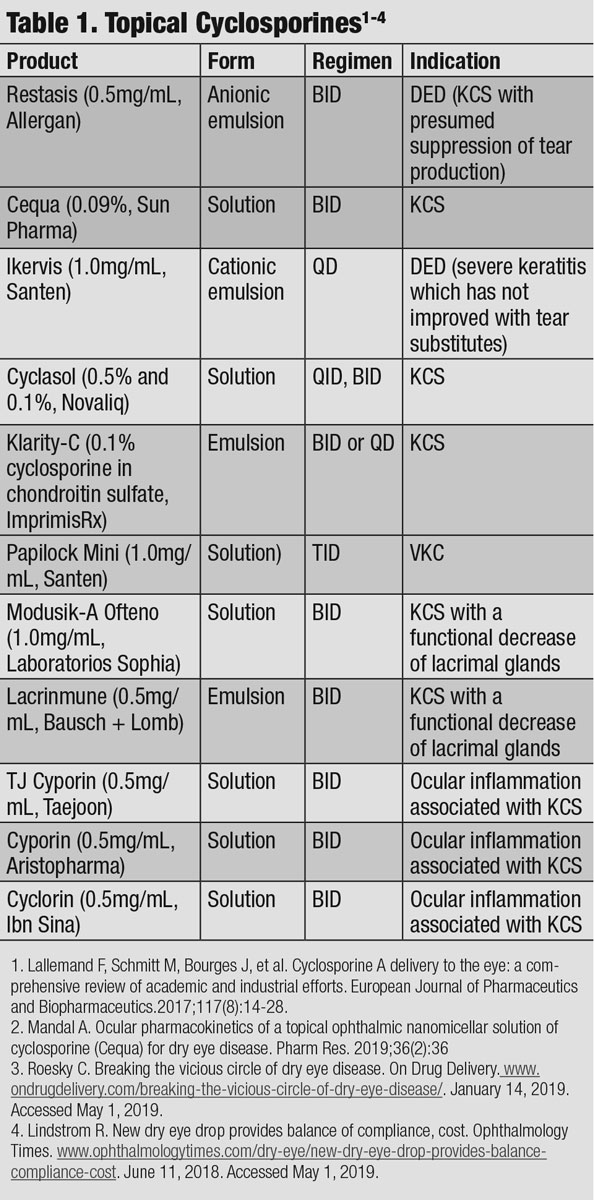 |
| Click table to enlarge. |
Future Directions
Additional research is being performed to evaluate the use of CsA-eluting contact lenses to treat dry eye.47 These lenses showed an initial burst and sustained release of CsA until 48 hours. New Zealand rabbits exhibited improved clinical parameters and conjunctival goblet cell density as well as decreased inflammatory cytokines. Advancements in drug delivery continue to be explored for the treatment of this multifactorial ocular disease.
The inflammatory nature of dry eye disease has been widely accepted; thus, the direction for treatment research is geared toward the reduction of inflammatory cytokines. Cyclosporine A inhibits the immune reaction. There are documented drawbacks to this drug in regards to its somewhat poor tolerability, instability and low bioavailability. Several pharmaceutical companies are working on formulations to maximize the effects while minimize side-effects and keep dosing infrequent.
The above formulations of cyclosporine differ in their concentration and preparation and thus bioavailability to ocular tissue. That being said, future clinical research may assist us in choosing the right medication for treatment of ocular surface inflammatory conditions by understanding if one formulation will provide greater improvement in ocular surface staining, increased tear production, as well as tolerability.
Dr. Hessen is a clinical instructor at the Wilmer Eye Institute’s Ocular Surface Diseases and Dry Eye Clinic at Johns Hopkins School of Medicine, where she specializes in ocular surface disease.
| 1. Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30(4):379-87. 2. Hawkes N. US’s $2bn annual spend on dry eye disease “brings tears to your eyes,” says critics. BMJ 2018;360:k492. 3. Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405-12. 4. Lemp MA, Baudouin C, Baum J, et al. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop (2007). Ocul Surf. 2007;5:75-92. 5. Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjogren’s syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950-3957. 6. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276-283. 7. Murphy CJ, Bentley E, Miller PE, et al. The pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogs. Invest Ophthalmol Vis Sci. 2011;52(6):3174-80. 8. Dastjerdi MH, Hamrah P, Dana R. High frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. 2009;28:1091-1096. 9. Kunert KS, Tisdale AS, Stern ME, et al. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000;118:1489–96. 10. Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjögren’s and non-Sjögren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–14. 11. Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–92. 12. Cejková J, Ardan T, Simonová Z, et al. Nitric oxide synthase induction and cytotoxic nitrogen-related oxidant formation in conjunctival epithelium of dry eye (Sjögren’s syndrome) Nitric Oxide. 2007;17:10–7. 13. Paul A, Wilson S, Belham CM, et al. Stress-activated protein kinases: activation, regulation and function. Cell Signal. 1997;9:403–10. 14. Pflugfelder SC, de Paiva CS, Tong L, et al. Stress-activated protein kinase signaling pathways in dry eye and ocular surface disease. Ocul Surf. 2005;3(Suppl 4):154–7. 15. Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–301. 16. Li DQ, Luo L, Chen Z, et al. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82:588–96. 17. Utine CA, Stern M, Akpek EK. Clinical review: topical ophthalmic use of cyclosporin A. Ocul Immunol Inflamm. 2010;18(5):352-61. 18. American Academy of Ophthalmology Cornea/External Disease Panel. Preferred Practice Pattern Guidelines. Dry Eye Syndrome. San Francisco, CA: American Academy of Ophthalmology. 19. Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47(2-3):199-125. 20. Stevenson W, Chauhan SK, Dana R. Dry Eye Disease: an immune-mediated ocular surface disorder. Arch Ophthalmology. 2012;130(1):90-100. 21. Pflugfelder SC, Wilhelmus KR, Osato MS, et al. The auto-immune nature of aqueous tear deficiency. Ophthalmology. 1986;93;1513-1517. 22. Stern ME, Gao J, Siemasko KF, et al. The role of the lacrimal gland functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78;409-416. 23. Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporine A ophthalmic emulsion in the treatment of moderate to severe dry eye disease: a dose-ranging, randomized trial. The Cyclospoine A Phase 2 Study Group. Ophthalmology. 2000;107:967-974. 24. Sall K, Stevenson OD, Mundorf TK, et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631-639. 25. Laibovitz RA, Solch S, Andriano K, et al. Pilot trial of cyclosporine 1% ophthalmic ointment in the treatment of keratoconjunctivitis sicca. Cornea. 1993;12;315-323. 26. Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120:330-337. 27. Benitez del Castillo JM, del Aguila A, Duran S. Influence of topically applied cyclosporine a in olive oil on corneal epithelium permeability. Cornea. 1994;13(2):136-40. 28. Sy A, O’Brein KS, Liu MP, et al. Expert opinion in the management of aqueous deficient dry eye disease (DED). BMC Ophthalmol. 2015;15:133. 29. Parrilha LR, Nai GA, Giuffrida R, et al. Comparison of 1% cyclosporine eye drops in olive oil and in linseed oil to treat experimentally-induced keratoconjunctivitis sicca in rabbits. Arq Bras Oftalmol. 2015;78(5):295-9. 30. Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr Opinion Ophthalmol. 2010;21(4):310-316. 31. Research in dry eye: report of the Research Subcommittee of the International Dry Eye Workshop (2007). The Ocular Surface. 2007;5(2):179-193. 32. Perry HD. Dry eye disease, pathophysiology, classification, and diagnosis. Am J Manag Care. 2008;14(3 Suppl):S79-87. 33. Sheppard JD, Donnenfeld ED, Holland EJ, et al. Effect of loteprednol etabonate 0.5% on initiation of dry eye treatment with topical cyclosporine 0.05%. Eye Contact Lens. 2014;40(5):289-96. 34. Mandal A, Gote V, Pal D, et al. Ocular pharmacokinetics of a topical ophthalmic nanomicellular solution of cyclosporine (Cequa) for dry eye disease. Pharm Res. 2019;36(2):36. 35. Luschmann C, Herrmann W, Strauss O, et al. Ocular delivery systems for poorly soluble drugs: an in-vivo evaluation. Int J Pharm. 2013;455(1–2):331–7. 36. Cholkar K, Gilger BC, Mitra AK. Topical, aqueous, clear cyclosporine formulation Design for Anterior and Posterior Ocular Delivery. Transl Vis Sci Technol. 2015;4(3):1. 37. Ashim K. Mitra PRV, Ulrich M. Grau Topical Drug Delivery Systems For Ophthalmic Use. In.: Aurinia Pharmaceuticals Inc Apr. 28, 2015. 38. Tauber J, Schechter BA, Bacharach J, Toyos MM, Smyth-Medina R, Weiss SL, et al. A phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease. Clin Ophthalmol. 2018;12:1921–9. 39. Jodi Luchs M. Phase 3 clinical results of cyclosporine 0.09% in a new nanomicellar ophthalmic solution to treatment keratoconjunctivitis sicca. International American Society of Cataract and Refractive Surgery (ASCRS) Annual Meeting Washington, DC; 2018. 40. Detuscu RM, Panfil A, Merkel UM, et al. Semifluorinated Alkanes as a liquid drug carrier system for topical ocular drug delivery. E J Pharm Biopharm 2014;88:123-8. 41. Agarwai O, Scherer D, Günther B, Rupenthal ID. Semifluorinated alkane based systems for enhanced corneal penetration of poorly soluble drugs. Int J Pharm 2018;538:119-29. 42. Wirta D, Torkildsen G, Moreira H, et al. A clinical phase 2 study to assess efficacy, safety and toleratbility of CyclASol for treatment of dry eye disease (DED). Ophthalmol. www.aaojournal.org/article/S0161-6420(18)32132-8/fulltext. November 11-14, 2017. Accessed May 1, 2019. 43. Leonardi A, Van Setten G, Amrane M, et al. Efficacy and Safety of 0.1% cyclosporine A cationic emulsion in the treatment of severe dry eye disease: a multicenter randomized trial. Eur J Ophthalmol 2016;26(4):287-296. 44. Baudoin C, Figueiredo FC, Messmer EM, et al. A randomized study of the efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of moderate to severe dry eye. Eur J Ophthalmol 2017;27(5);520-30. 45. Matossian C, Trattler WB, Loh JM. Tolerability and Efficacy of topical 0.1% cyclosporine in chondroitin sulfate ophthalmic emulsion: an open-label registry study. ASCRS annual meeting 2019. 46. Moon JW, Lee H-J, Shin KC, et al. Short term effectsof topical cyclosporine and viscoelastic on the ocular surfaces in patients with dry eye. Korean J Ophthalmol 2007;21(4):189-94. 47. Choi JH, Li Y, Shrestha T, et al. The efficiency of cyclosporine A-eluting contact lenses for the treatment of dry eye. Curr Eye Research. 2018;44(5):486-96. |

