It was long theorized that regions of ischemic retina released some type of unknown factor that promoted growth of new vasculature (i.e., neovascularization). The molecule now known as vascular endothelial growth factor (VEGF) was discovered and found to be present in much higher concentrations in eyes with neovascularization than in those without. Animal models also revealed that artificially creating a hypoxic retina led to increased VEGF and that injecting VEGF into the eye induced iris neovascularization and neovascular glaucoma.1
The development of anti-VEGF injections has provided a medical option to patients with certain posterior segment issues that simply didn’t exist before. New compounds continue to be developed and tested to better resolve retinal issues.
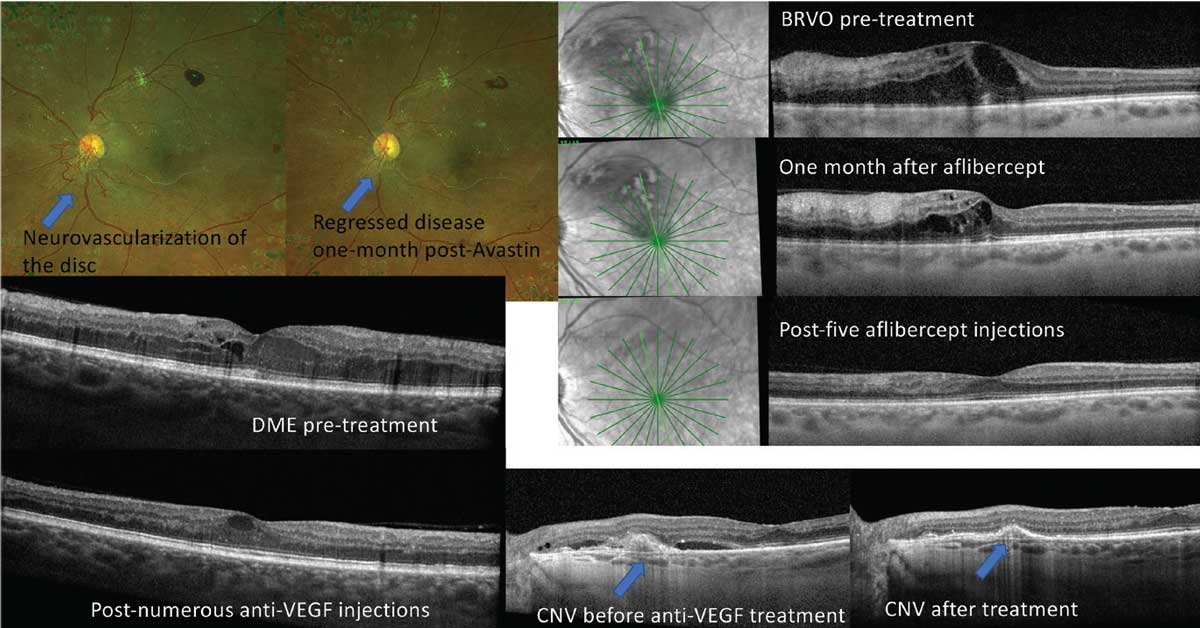 |
| Fig 1. Anti-VEGF injections are beneficial for a wide array of posterior segment complications. Click image to enlarge. |
A New Treatment Emerges
In the early 2000s, innovation leapt forward and anti-VEGF compounds began to be used therapeutically. The first was intravenous bevacizumab (Avastin, Genentech), which was FDA-approved for the treatment of colon cancer in February 2004. Soon after, an anti-VEGF molecule was approved for the eye: pegaptanib (Macugen, originally Eyetech/Pfizer, now Bausch + Lomb), approved in December 2004 for treatment of neovascular age-related macular degeneration (wet AMD).
Pegaptanib’s success was short-lived, as the much more affordable off-label use of bevacizumab (reconstituted for intravitreal injection by a compounding pharmacy) and the 2006 FDA approval of Genentech’s on-label wet AMD drug ranibizumab (Lucentis) both eclipsed Macugen in clinical efficacy.1
In 2011, aflibercept (Eylea, Regeneron) would be approved for wet AMD based on the results of the VIEW trials that showed aflibercept dosed every two months was not inferior to ranibizumab dosed monthly.2 Ranibizumab and aflibercept would later be approved for the treatment of diabetic macular edema (DME) and macular edema from retinal vein occlusions (RVO). In addition, ranibizumab is approved for the treatment of myopic choroidal neovascularization (CNV). Off-label use of bevacizumab remains widespread due to its significantly lower cost.
During this anti-VEGF revolution, wet AMD went from a nearly untreatable sentence of blindness to a much more manageable condition with early intervention. In addition, patients with diabetic retinopathy (DR) and RVO had new hope of vision-improving therapies that didn’t rely on destructive laser treatments (Figure 1). Some might have thought that the rest would be history, but it was just getting started.
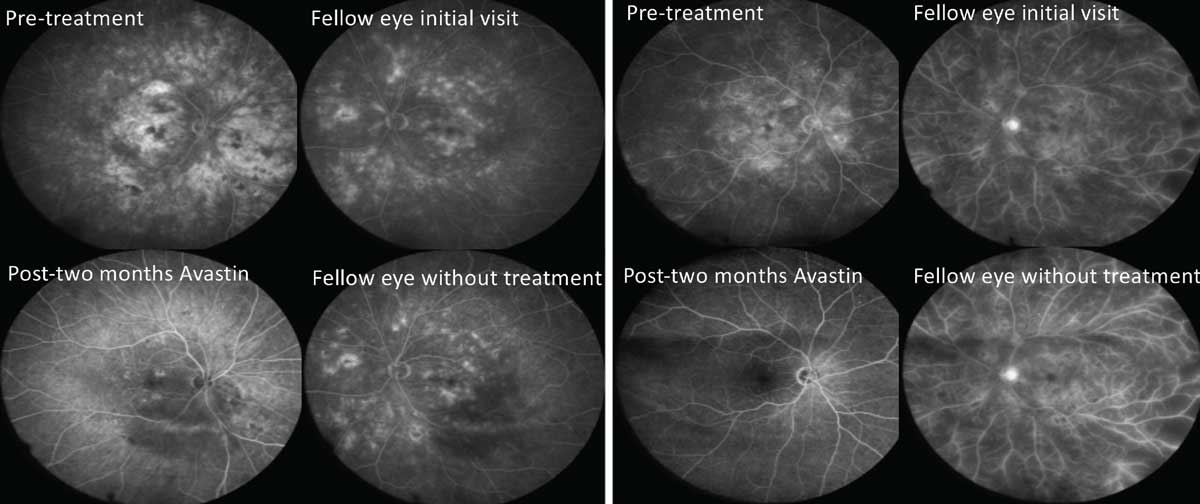 |
| Fig. 2. Periodic treatment with anti-VEGF has been shown to reverse severity of retinopathy, a benefit beyond simply treating DME and proliferative disease. This figure shows fluorescein angiographies of two separate patients with severe NPDR. The OD of each patient was treated with two monthly injections, while the OS was monitored. Notice the profound improvements in the treated eyes vs. lack of improvement in the untreated fellow eyes. Click image to enlarge. |
Ranibizumab and Aflibercept
Regarding DR treatment, anti-VEGF was primarily used for many years to treat DME per ranibizumab and aflibercept’s FDA approval. Panretinal photocoagulation (PRP) was still the mainstay treatment for proliferative DR (PDR), and patients with non-proliferative DR (NPDR) without macular edema were predominantly monitored without treatment.
Observation of patients who were receiving injections anecdotally showed regression of retinal and iris neovascularization and general improvement of the retinopathy as a whole, which gave rise to the following questions:
(1) Can we treat patients with proliferative disease with anti-VEGF alone, and how does this stack up to treatment with PRP?
(2) If we treat patients without proliferative disease and without DME prophylactically with anti-VEGF, can we reverse retinopathy and prevent sight-threatening complications?
DRCR Protocol S compared the safety and efficacy of ranibizumab with PRP for the treatment of PDR. Ranibizumab was found to be noninferior to PRP, and secondary outcomes showed superior visual acuity gains and less visual field loss when using ranibizumab over PRP. In addition, patients in the ranibizumab arm were less likely to develop center-involving DME.3
The downside of ranibizumab over PRP is increased treatment burden, with ranibizumab patients requiring much more frequent treatment than those receiving PRP. Patient compliance therefore remains a chief concern. If patients do not comply with frequent office visits and injections, there is risk of disease progression and blindness. The decision to treat a PDR patient with anti-VEGF, PRP or a combination of both remains very patient-specific for most physicians.3
The PANORAMA trial evaluated the use of aflibercept vs. sham injection in patients with moderately severe to severe nonproliferative diabetic retinopathy without macular edema. The primary endpoint was a two-step regression on the DR severity scale (DRSS), and secondary endpoints were proportion of patients developing vision-threatening complications such as development of retinal or iris neovascularization and center-involving DME.
The study had three treatment arms: aflibercept dosed q16 weeks after three initial monthly doses and one eight-week interval dose, aflibercept dosed q8 weeks after five initial monthly loading doses with PRN dosing after week 56 and sham injections (control group).
One-year results showed that 80% of patients in the q8 week arm, 65% of patients in the q16 week arm and 15% of patients receiving sham met the primary endpoint of at least a two-step reduction on the DRSS. In addition, patients in both the q8 and q16 week arms had significant reduction in their risk of developing vision-threatening complications and center-involving diabetic macular edema. Two-year results showed continued benefit of fixed-interval injections in this patient population.4
The results of Protocol S and the PANORAMA study resulted in new approvals for ranibizumab and aflibercept. Ranibizumab was approved in 2017 for all forms of DR, and then aflibercept followed with the same approval in 2019. These results and approvals show the potential for disease regression and suggest a benefit for earlier intervention for severe NPDR patients without macular edema, shifting the paradigm towards earlier referral and treatment. The use of anti-VEGF for this patient group, who traditionally has been monitored, is still very patient- and physician-dependent (Figure 2).
Novel Drugs and Targets
For about a decade, these three drugs—bevacizumab, ranibizumab and aflibercept—remained our only anti-angiogenic weapons in the treatment arsenal. The pursuit continues for novel drugs with increased durability, efficacy and even lower cost to ultimately improve patient visual outcomes and decrease treatment burdens.
At present, there is another influx of innovation and potential occuring, with two new anti-VEGF agents (brolucizumab and faricimab) and a new delivery method for ranibizumab (Susvimo) all approved since 2019, and numerous others in the pipeline. Some of these try to revamp what is already available while others seek to inhibit new targets with entirely new mechanisms of action.
Beovu. The 2019 approval of Beovu (brolucizumab, Novartis) gave patients access to four options for anti-VEGF therapy. The strong clinical trial outcomes through all phases of study led many providers to predict Beovu would grab a significant market share in the space. Why did that not happen? With expanded usage, providers began noticing incidents of intraocular inflammation. Most were mild and self-resolving, but some exhibited signs of severity, including branch artery occlusions and vitritis.5 These patients suffered vision loss as a result. This led to an extensive investigation, multiple presentations at national meetings and many new publications all directly dealing with the issue of intraocular inflammation and Beovu.6,7
The overall incidence of intraocular inflammation was found to be around 4%.7 This would be considered an acceptable risk for most agents, but when compared with what amounts to no intraocular inflammation risk with Avastin, Eylea and Lucentis, Beovu’s adoption faltered and most providers stopped using it. This has left it as the option of last resort for most retina specialists, which is reflected in its diminished market share and its limited commercial prospects going forward.
The authors of this article continue to advocate for its use as it seems to be the strongest available agent and is the best option for patients with an under-response to Eylea. When choosing to use Beovu in a patient, a more extensive informed consent process is advisable with full disclosure of the increased risk of intraocular inflammation compared with other agents. We have notably not seen a case of intraocular inflammation in our practice for over two years despite continued significant use in our practice.
Conbercept (Chengdu Kanghong Biotechnology). This agent has been approved by the Chinese FDA since 2013 for treatment of wet AMD, making it one of the most widely used anti-VEGF therapies for this condition worldwide. Conbercept is a recombinant fusion protein composed of the second IgG domain of VEGFR1 and the third and fourth domains of VEGFR2 to the constant region (Fc) of human IgG1.
There are more than 30 clinical trials regarding conbercept for Chinese and the United States FDA approval. These trials concern the international market for wet AMD, non-AMD choroidal neovascular membranes, DR and RVO macular edema, and retinopathy of prematurity. Trials also evaluate the drug in combination with systemic chemotherapy for treatment of retinoblastoma.8 There are also studies on treatment of neovascular glaucoma and macular edema secondary to uveitis.
The Aurora, Phoenix and Panda clinical trials have evaluated various dosing (0.5mg vs. 2mg) and treatment regimens such as three loading doses followed by monthly, every eight to 12 weeks or PRN. All these regimens have indicated this recombinant anti-VEGF is an effective treatment for wet AMD.9-12
Conbercept’s potential benefit is its higher binding affinity than other anti-VEGF drugs (30x higher than Lucentis and Avastin). Clinical trial results will help to reveal how this translates to real-world treatment. Thus far it is comparable to other currently commercially available anti-VEGF in clinical trials; therefore, this would also likely be another available agent in the United States and other countries for treatment of wet AMD and other conditions in which this class of drugs have been successfully used over nearly two decades.13 We cross our fingers for its approval in the US.
Faricimab (Vabysmo, Genentech). This is a bispecific antibody that targets both vascular endothelial growth factor A (VEGF-A) and angiopoietin-2 (Ang-2). The latter is a growth factor involved in the angiogenesis pathway. It is upregulated by hypoxia and is present in increased levels in eyes with DR and wet AMD.14 Faricimab has been evaluated thus far across four Phase III trials (TENYA and LUCERNE for wet AMD and YOSEMITE and RHINE for DME) showing noninferior visual gains vs. aflibercept. Of great interest, about half of patients receiving faricimab could successfully extend treatment to four months. Use of faricimab for the treatment of macular edema from RVO is currently being evaluated in the Phase III trials COMINO and BALATON.15 In January 2022, the FDA granted approval for Vabysmo for wet AMD and DME treatment.16
Byooviz (ranibizumab-nuna, Samsung Bioepis). This medication is a biosimilar of ranibizumab; that is, a biologic medical product highly similar to an already FDA-approved agent that has been shown to be equally safe and effective. A biologic is a medication that is manufactured in, synthesized from or extracted from a biological source. This differs from a traditional drug that is created through chemical synthesis and not through use of biological sources. Biosimilars can be compared with a generic form of a traditional, chemically synthesized drug. Byooviz was approved by the FDA in September 2021 for the treatment of wet AMD, macular edema from RVO and myopic CNV. It has not been approved for treatment of DME or DR.17
Byooviz’s approval came after a Phase III trial of 705 patients, 634 of whom continued treatment into week 48, showed similar efficacy and safety between Byooviz and ranibizumab. Concerns regarding Byooviz center primarily around safety. Although biosimilars are comparable to generics regarding biologics, the manufacturing process of biologic medical products may be more important in the safety and efficacy of the drug than for non-biologics. In addition, there is concern that unsuspected adverse effects may be uncovered when going from use in hundreds of patients in a clinical trial to thousands in real-world treatment.17
The main advantage of the Byooviz will likely be cost, allowing patients to potentially get the benefits of ranibizumab without the high price tag, increasing patient access and lowering healthcare cost. The exact cost of the medication is still unknown, and it is not planned to be released in the US until June 2022.
While the concept of biosimilars is somewhat unfamiliar to many physicians, one survey’s results suggested most physicians would consider using an anti-VEGF biosimilar once available for their patients. With numerous other companies moving forward with additional ranibizumab and even aflibercept biosimilars, time will ultimately tell how readily the medications will be accepted and incorporated into practice.18,19
Ocular-specific bevacizumab. Outlook Therapeutics is a biopharmaceutical company in late clinical trial stages anticipated to launch ONS-5010, the first commercially available bevacizumab, under the tradename of Lytenava, for treatment of wet AMD, DME and branch RVO (BRVO).20,21
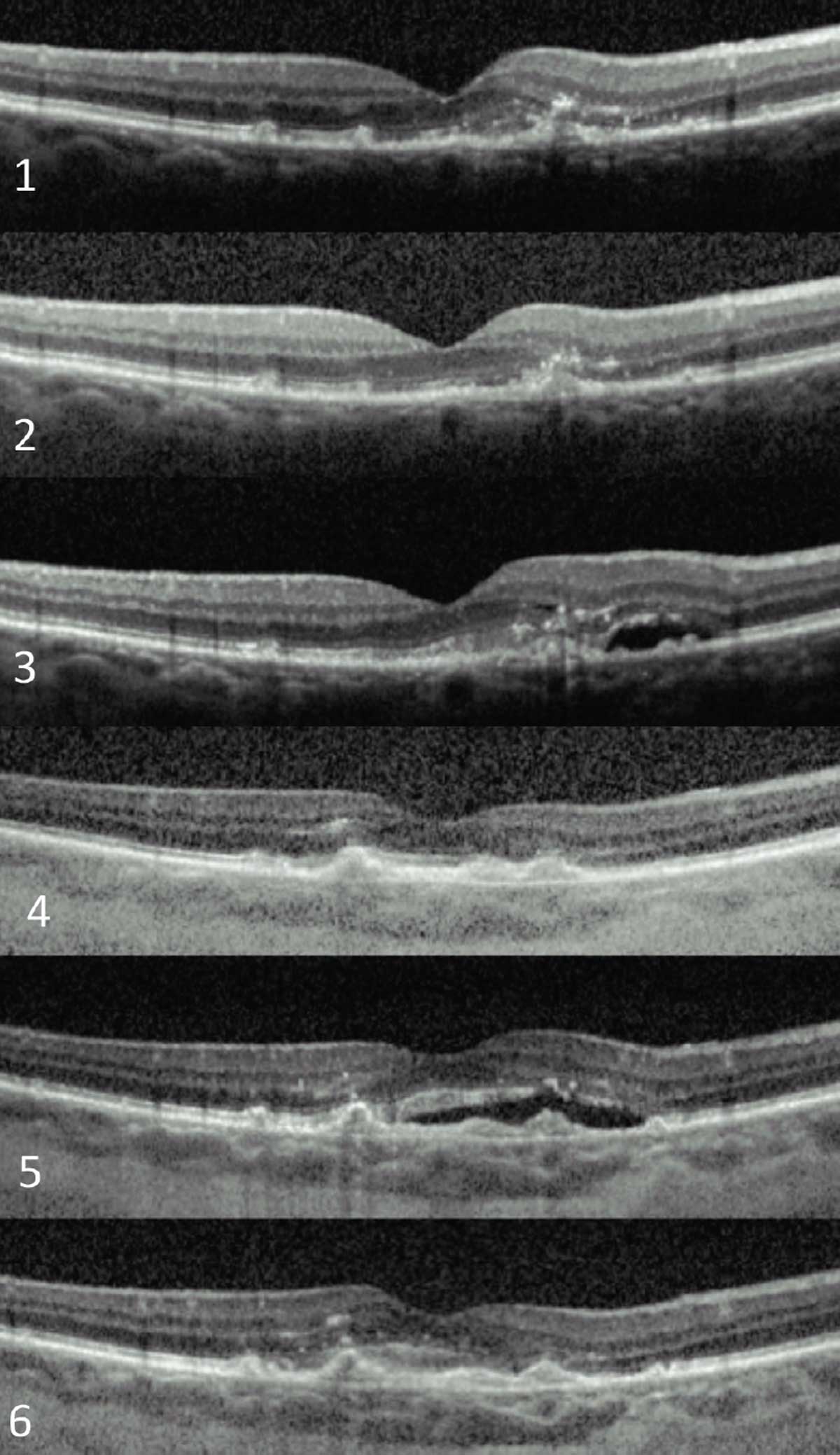 |
| Fig. 3. An exudative AMD patient who has required over 20 anti-VEGF injections in nine years to maintain 20/40 visual acuity. These are a few snapshots in time during the course of her treatment. Click image to enlarge. |
The NORSE noninferiority clinical trials have been comparing ONS-5010 with ranibizumab indications of the above-mentioned conditions. Outlook is hoping to launch this product as a potential first-line anti-VEGF therapy based on historical use of anti-VEGF, particularly off-label bevacizumab, for ophthalmic conditions over nearly two decades. The introduction of an FDA-approved commercially available bevacizumab for ophthalmic use has the potential to reduce consumer confusion about off-label drug use, reduce chance of adverse events, particularly endophthalmitis, offer better quality control and shift the burden of product liability. However, much of this will be seen in the future based on price point, insurance coverage and the expanded novel and biosimilar agents.
Novel Delivery Systems
Despite all the success with anti-VEGF therapy, the downside of the high burden of treatment—six or more intravitreal injections per year—remains an obstacle (Figure 3). Patients are still tied to frequent office visits and need for recurrent treatments that, while tolerable, many find uncomfortable to some extent and inconvenient to attend to (often, a family member must accompany the patient, increasing inconvenience and disruption).
Longer-acting and more efficacious drugs as described above may be part of the solution for this problem, but additionally advances in medication delivery are likely to be a game changer. These novel delivery systems try to address the question of how we can get these sight-saving medications into the eye without subjecting our patients to frequent, sometimes life-long, injection regimens.
Susvimo (Roche). This was an important FDA approval in the retina space for 2021—a refillable, implantable delivery system for sustained release of ranibizumab. It is currently approved for wet AMD and has ongoing clinical trials for DME and DR.22 It delivered on its promise of sustained treatment effect with a much lower burden of care across all trial phases for wet AMD. It showed equal visual gains as ranibizumab injected monthly, and over 98% of patients with Susvimo could go six months before requiring a refill (Figure 4).23
The procedure itself evolved as it progressed through the approval process. Early problems with vitreous hemorrhage were reduced with more meticulous surgical techniques centered around hemostasis and wound creation. The grain of rice–sized implant is not sutured in place but instead fits snugly inside the eye with only a minimally elevated flange resting on the external sclera. This allows for easy refills in the clinic at six-month intervals through the small central septum visible underneath the semitransparent conjunctiva and Tenon’s capsule.22
Rollout is limited to start with only the principal investigators at certain sites performing the first commercial implantations. John Kitchens, MD, was the first to implant one commercially, and our site in Memphis was the first to implant Susvimo in Tennessee a week later.
Clinical trial results indicate a good safety profile with an approximate 2% endophthalmitis risk, most of which was linked to conjunctival retraction. Implant dislocations were rare as well.23 The risk of these two problems is dramatically reduced by employing good surgical practices including perfect wound creation and meticulous closure of conjunctiva and tenons. This is an implant designed to last the entirety of the patient’s lifetime with near infinite refill ability. It is a good option for active patients struggling with the burden of frequent office visits and injections.
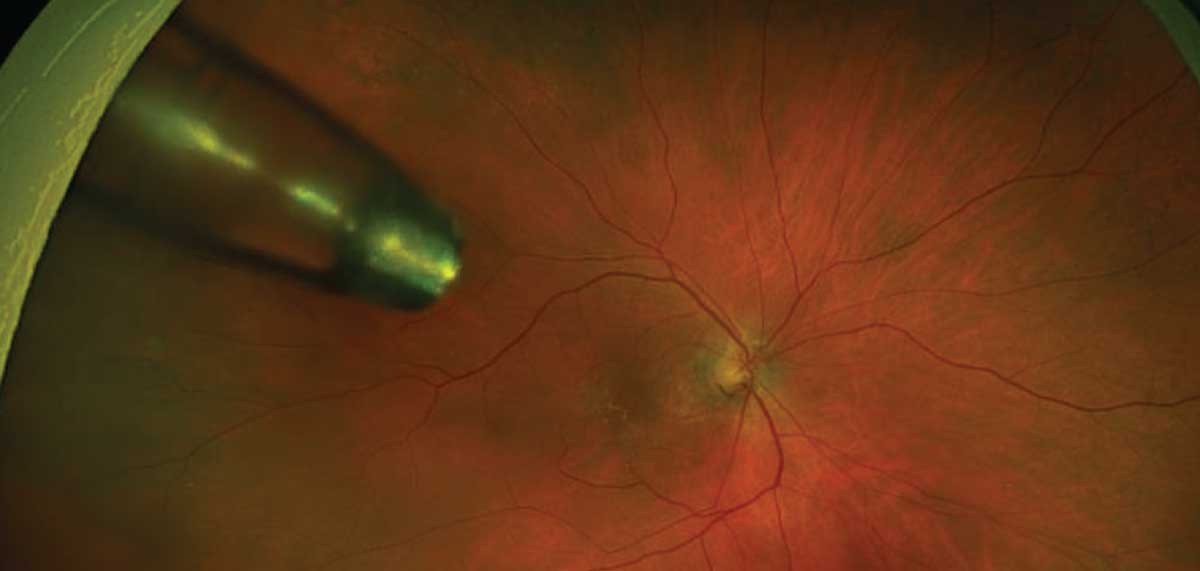 |
| Fig. 4. Ultra-widefield image of wet AMD patient treated with the Susvimo implant. Click image to enlarge. |
Gene Therapy Delivery
There are a multitude of active gene therapy trials currently underway in the retina space. We will focus on one company that is showing great promise. RegenxBio has multiple gene therapy trials underway for wet macular degeneration. The biologic agent under study is a modified adenoviral vector containing a genetic package that, through the process of transfection, enables retinal pigment epithelial cells to produce a modified ranibizumab-like molecule that then dramatically reduces the number of intravitreal injections a patient requires.24,25
Public results so far are outstanding, with a clinically significant reduction in intravitreal injections given for those dosed via vitrectomy and subretinal delivery and a clinically significant reduction in intravitreal injections with those dosed via an in-office suprachoroidal procedure.24,25
The vitrectomy-based procedure requires great skill and a fine touch but is able to be performed with off-the-shelf vitrectomy supplies. The suprachoroidal in-office procedure requires the use of a novel suprachoroidal delivery syringe with a specially designed needle tip to safely enter the suprachoroidal space. Both procedures are exceptionally well-tolerated, with only a few patients exhibiting non-severe inflammation following treatment.24,25 These treatments have the potential to alleviate VEGF-driven diseases while at the same time dramatically decreasing the frequency of intravitreal injections.
In addition to RegenxBio, the company Adverum Biotechnologies is using a viral vector intravitreally for expression of aflibercept with positive Phase I results, now moving into Phase II. Numerous gene therapy trials with various other anti-angiogenic targets are also underway.26
Long-term Effects
Anti-VEGF therapy in many patients diagnosed with wet AMD, DME and macular edema due to RVO can turn into long-term treatment, as these are chronic conditions needing chronic care. Studies and experience have shown us that gaps and lapses in therapy can have detrimental consequences and result in poor outcomes.27 However, long-term invasive therapy of any medical condition can have its own set of consequences and setbacks. These include reduced patient’s adherence to recommended treatment regimen due to a variety of reasons and barriers, including cost, increased chance of adverse events and reduced effectiveness of therapy (tachyphylaxis).
In regards to long-term and multiple intravitreal injections, adverse events and tachyphylaxis are also possible but are not great concerns. With regards to adverse events, endophthalmitis is the greatest concern, the incidence of which is approximately one in 3,000 injections (0.033%).28 Ocular inflammation and occlusive vasculitis has been reported with these injections and particularly became a concern with repeated injections of brolucizumab.7,29,30 This resulted in an FDA label update in June 2020 warning prescribers.
Tachyphylaxis has also been noted with intravitreal injections. This is an uncommon finding in treatment of wet AMD in a minority of patients.31 Cases of recalcitrant DME as well as macular edema associated with RVO are also encountered clinically, in which case alteration of medication to other anti-VEGF agents or to intravitreal steroidal agents was considered to alter the course of the disease.32-35
Clinical Takeaways
Intravitreal delivery of anti-VEGF medications has dramatically improved visual outcomes of patients suffering from numerous retinal pathologies. The use of some widely accepted drugs has been going on for over 15 years now, but new possibilities and innovations continue to expand options for patients and physicians, allowing for better patient access, improving efficacy and easing the burden of treatment.
Dr. Haynes is a consultative optometrist at the Charles Retina Institute in Germantown, TN, and consulting faculty at Southern College of Optometry in Memphis, TN. She is a Fellow of the American Academy of Optometrists (AAO). She is a paid speaker for Heidelberg Engineering and a consultant for Notal Vision. Dr. Huddleston is a vitreoretinal surgeon at the Charles Retina Institute. He has no financial disclosures. Dr. Rafieetary is a consultative optometric physician at the Charles Retina Institute. He is a Fellow of the AAO. Dr. Rafieetary is on advisory boards for Heidelberg Engineering, Optos, Regeneron, Notal Vision and Cardinal Health.
1. Kim LA, D’Amore PA. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol. 2012;181(2):376-9. 2. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-48. 3. Sun JK, Glassman AR, Beaulieu WT, et al. Rationale and application of the Protocol S anti–vascular endothelial growth factor algorithm for proliferative diabetic retinopathy. Ophthalmology. 2019;126(1):87-95. 4. Brown DM, Wykoff CC, Boyer D, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139(9):946-55. 5. Witkin AJ, Hahn P, Murray TG, et al. Occlusive retinal vasculitis following intravitreal brolucizumab. J Vitreoretin Dis. 2020;4(4):269-79. 6. Baumal CR, Bodaghi B, Singer M, et al. expert opinion on management of intraocular inflammation, retinal vasculitis and vascular occlusion after brolucizumab treatment. Ophthalmol Retin. 2021;5(6):519-27. 7. Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis and retinal occlusion–related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128(7):1050-9. 8.Conbercept list results. ClinicalTrials.gov. clinicaltrials.gov/ct2/results?cond=conbercept&term=&cntry=&state=&city=&dist=. Accessed December 29, 2021. 9. Li X, Xu G, Wang Y, et al. AURORA study group. Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized Phase II study: AURORA study. Ophthalmology. 2014;121(9):1740-7. 10. Liu K, Song Y, Xu G, et al; PHOENIX study group. Conbercept for treatment of neovascular age-related macular degeneration: results of the randomized Phase III PHOENIX study. Am J Ophthalmol. 2019;197:156-67. 11. Zhang J, Liang Y, Xie J, et al. Conbercept for patients with age-related macular degeneration: a systematic review. BMC Ophthalmol. 2018;18(1):142. 12. Li X, Luo H, Zuo C, Zhang Z, Zhang J, Zhang M. Conbercept in patients with treatment-naive neovascular age-related macular degeneration in real-life setting in China. Retina. 2019;39(7):1353-60. 13. Sacconi R, Giuffrè C, Corbelli E, et al. Emerging therapies in the management of macular edema: a review. F1000Res. 2019;8:1413. 14. Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019;8(5):471. 15. FDA accepts application for Roche’s faricimab for the treatment of neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME). Roche. www.roche.com/investors/updates/inv-update-2021-07-29b.htm. Accessed December 29, 2021. 16. FDA approves Genentech’s Vabysmo, the first bispecific antibody for the eye, to treat two leading causes of vision loss [press release]. San Francisco,CA: Genentech; January 28, 2022. www.gene.com/media/press-releases/14943/2022-01-28/fda-approves-genentechs-vabysmo-the-firs-. Accessed January 29, 2022. 17. FDA approves first biosimilar to treat macular degeneration disease and other eye conditions. FDA. www.fda.gov/news-events/press-announcements/fda-approves-first-biosimilar-treat-macular-degeneration-disease-and-other-eye-conditions. Accessed December 29, 2021. 18. RP Staff. First ranibizumab biosimilar approved. Rev Ophthalmol. 2021;28(10):5,9. 19. Blank C. AAO spokesperson: FDA approval of Byooviz, first biosimilar to Lucentis, Is “significant.”Managed Healthcare Executive. www.managedhealthcareexecutive.com/view/aao-spokesperson-fda-approval-of-byvooviz-first-biosimilar-to-lucentis-is-significant-. October 7, 2021. Accessed December 29, 2021. 20. Oskouei ST. Opinion: Is the ophthalmology market ready to embrace biosimilars? Center for Biosimilars. www.centerforbiosimilars.com/view/is-the-ophthalmology-market-ready-to-embrace-biosimilars-. January 16, 2021. Accessed December 29, 2021. 21. Outlook Therapeutics submits IND application for ONS-5010 to the FDA [news release]. Cranbury, NJ: Outlook Therapeutics; March 1, 2019. ir.outlooktherapeutics.com/node/8016/pdf. Accessed December 29, 2021. 22. Outlook Therapeutics announces FDA acceptance of IND for ONS-5010 [news release]. Cranbury, NJ: Outlook Therapeutics; April 1, 2019. www.globenewswire.com/news-release/2019/04/01/1790614/0/en/ outlook-therapeutics-announces-fda-acceptance-of-ind-for-ons-5010.html. Accessed December 29, 2021. 23. FDA approves Genentech’s Susvimo, a first-of-its-kind therapeutic approach for wet age-related macular degeneration (AMD) [press release]. San Francisco,CA: Genentech; October 22, 2021. www.gene.com/media/press-releases/14935/2021-10-22/fda-approves-genentechs-susvimo-a-first-. Accessed January 2, 2022. 24. Holekamp NM, Campochiaro PA, Chang MA, et al. Archway Randomized Phase 3 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2022;129(3):295-307. 25. RegenxBio announces additional positive long-term and interim Phase I/IIa trial update for RGX-314 for the treatment of wet AMD [news release]. Rockville, MD: RegenxBio; April 22, 2020. regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-announces-additional-positive-long-term-and-interim. Accessed December 29, 2021. 26. Koponen S, Kokki E, Kinnunen K, Ylä-Herttuala S. Viral-vector-delivered anti-angiogenic therapies to the eye. Pharmaceutics. 2021;13(2):219. 27. RegenxBio presents additional positive interim data from trials of RGX-314 in wet AMD and diabetic retinopathy using suprachoroidal delivery at AAO 2021 [news release]. Rockville, MD: RegenxBio; November 12, 2021. regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-presents-additional-positive-interim-data-trials-rgx. Accessed January 2, 2022. 28. Greenlee TE, Wang VY, Kang H, et al. Consequences of lapses in treatment with vascular endothelial growth factor inhibitors in neovascular age-related macular degeneration in routine clinical practice. Retina. 2021;41(3):581-7. 29. Li AL, Wykoff CC, Wang R, et al. Endophthalmitis after intravitreal injection: Role of prophylactic topical ophthalmic antibiotics. Retina. 2016;36(7):1349-56. 30. Haug SJ, Hien DL, Uludag G, et al. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Reports. 2020;18:100680. 31. Jain A, Chea S, Matsumiya W, et al. Severe vision loss secondary to retinal arteriolar occlusions after multiple intravitreal brolucizumab administrations. Am J Ophthalmol Case Reports. 2020;18:100687. 32. Hara C, Wakabayashi T, Fukushima Y, et al. Tachyphylaxis during treatment of exudative age-related macular degeneration with aflibercept. Graefe’s Arch Clin Exp Ophthalmol. 2019;257(11):2559-69. 33. Chakraborty D, Sheth JU, Boral S, Sinha TK. Off-label intravitreal brolucizumab for recalcitrant diabetic macular edema: a real-world case series. Am J Ophthalmol Case Reports. 2021;24:101197. 34. Rishi P, Rishi E, Kuniyal L, Mathur G. Short-term results of intravitreal dexamethasone implant (Ozurdex) in treatment of recalcitrant diabetic macular edema: a case series. Oman J Ophthalmol. 2012;5(2):79-82. 35. Klein KA, Cleary TS, Reichel E. Effect of intravitreal aflibercept on recalcitrant diabetic macular edema. Int J Retin Vitr. 2017;3(1):16. |

