 A 36-year-old male presents following the development of painful vision loss O.S. over several days. He has worn contact lenses for the past several years without incident. This vision loss initially began as a purulent discharge that he just wiped away.
A 36-year-old male presents following the development of painful vision loss O.S. over several days. He has worn contact lenses for the past several years without incident. This vision loss initially began as a purulent discharge that he just wiped away.
Best-corrected visual acuity is 20/400 O.S. Biomicroscopy shows a large paracentral infiltration with overlying epithelial ulceration and profound corneal edema. The corneas thickness is thinned by one-third, and the visual axis is threatened. There is also a heavy anterior chamber reaction.
Clearly, the patient has microbial keratitis, presumably bacterial in nature. Due to the threat both to the visual axis and of perforation, cultures are obtained. Still, the patient will need strong bacterial antibiosis before the results are availablebut through what modality?
Opinions differ on the management of bacterial corneal ulcers. Currently, both fourth-generation fluoroquinolones and fortified antibiotics are used to manage this condition. Many optometrists and ophthalmologists employ fourth-generation fluoroquinolones off-label for non-vision-threatening cases and use fortified antibiotics when vision-threatening ulceration presents. Corneal specialists tend to prefer fortified antibiotics as their first treatment of choice; but, keep in mind that corneal specialists typically see patients who have already failed on commercially available medications.
Fourth-generation fluoroquinolones are popular in the antibiotic arena, but fortified antibiotics are often relied upon and have long been the standard treatment for sight-threatening bacterial ulcers. How exactly did we arrive at this standard of care?
In this months column, we neither advocate nor vilify the use of fortified antibiotics in the management of bacterial keratitis. Instead, we will examine the science behind this standard of care.
Challenges of Fortified Antibiotics
The use of fortified antibiotics for bacterial keratitis involves the compounding of any number of antibiotics from concentrations designed for systemic use and the diluting of antibiotics originally meant for intravenous use. Fortified cefazolin and vancomycin are reconstituted with 0.9% sodium chloride and artificial tears. Gentamicin and tobramycin are prepared by adding parenteral forms into their commercial ophthalmic solutions.
There are some challenges in the use of fortified antibiotics. For example, they are only attainable from a pharmacy that does compounding. And, because these agents are not commercially prepared, there is a degree of instability and risk of contamination. Topical fortified antibiotics used for longer than seven 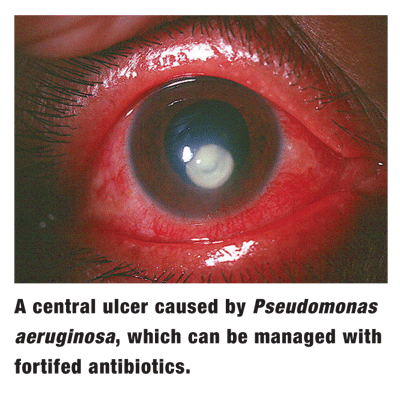 days should be stored at 4 C (39 F); those stored at higher than 24 C (75 F) should be discarded after seven days.1 Also, these medications can be very detrimental to the cornea.2 We have seen significant corneal toxicityand even periorbital contact dermatitisfrom the use of fortified antibiotics for the management of bacterial keratitis.
days should be stored at 4 C (39 F); those stored at higher than 24 C (75 F) should be discarded after seven days.1 Also, these medications can be very detrimental to the cornea.2 We have seen significant corneal toxicityand even periorbital contact dermatitisfrom the use of fortified antibiotics for the management of bacterial keratitis.
Typically, two medications must be used alternately for optimal bacterial coverage. But, a recent study suggests that medications could be combined with the same potency and stable physical properties.3
Anointing a Standard of Care
We have spoken to many colleagues on this subject. What surprises us: the number who believe that fortified antibiotics are FDA approved for management of bacterial keratitis. Many of these doctors do not realize that these agents never received FDA approval for the treatment of corneal ulcers.
Nevertheless, there is compelling scientific evidence regarding the
efficacy of fortified antibiotics in the management of bacterial keratitis.4-10 For many years, the use of fortified antibiotics represented the standard of care for patients with bacterial keratitis. But, this standard first developed through anecdotal reports of success and case series of patients treated with fortified antibiotics.
What had always been missing was a controlled comparison between fortified antibiotics and commercially available agents in the management of bacterial keratitis. So, fortified antibiotics were not actually proven to be the superior choice; rather, they were anointed as standard of care under the philosophy that more must be better.
In the 1990s, the development of topical fluoroquinolones offered an alternative management option for patients with bacterial keratitis. And, for the first time, commercially available antibiotics were scientifically compared to fortified antibiotics.
The Bacterial Keratitis Study Group found that the third-generation fluoroquinolones (ofloxacin and ciprofloxacin, regarded by some as second generation) were equivalent to fortified cefazolin and tobramycin solutions in the management of patients with bacterial keratitis.11-13 Also, the reduced frequency of ocular toxic effects and the relative ease of use of these fluoroquinolones were seen as additional benefits. This research indicated that the tested fluoroquinolones were as efficacious as the gold standard of fortified antibiotics; an alternate view could be that fortified antibiotics were seen to be no more effective than the commercial antibiotics tested in the study.
A Role for Fortified Antibiotics Today?
Since the results of the Bacterial Keratitis Study were published, bacteria have become resistant to third-generation fluoroquinolones.5,14,15 Most bacterial keratitis treatment failures that occurred have been with the use of ofloxacin and ciprofloxacin, and in these instances, fortified antibiotic treatment has been instituted successfully.5,7,15,16
The second major change in antibiotic therapy has been the development of fourth-generation fluoroquinolones. These have achieved much anecdotal and reported success in the management of bacterial keratitis.17,18 But, there are no published comparisons between fourth-generation fluoroquinolones and fortified antibiotics, so the benefits of fourth-generation fluoroquinolones compared to those of fortified antibiotics in the management of bacterial keratitis remain undetermined.
Where does this leave fortified antibiotics? They maintain a significant role in the management of severe, sight-threatening cases of bacterial keratitis; many clinicians prefer to fall back on this standard treatment. But, there are challenges involved in their use, and there is only scant scientific evidence comparing them to commercially available agents.
Many eye doctors share the misconception that fortified antibiotics underwent rigorous study years ago and were proven to be the most effective treatment for bacterial keratitis. Rather, they were believed to be the best option to combat a sight-threatening disease and were anointed the standard of care.
1. Arici MK,
2. Lin CP, Boehnke M. Effect of fortified antibiotic solutions on corneal epithelial wound healing. Cornea 2000 Mar;19(2):204-6.
3. Lin JM, Tsai YY, Fu YL. The fixed combination of fortified vancomycin and amikacin ophthalmic solution--VA solution: in vitro study of the potency and stability. Cornea 2005 Aug;24(6):717-21.
4. Erjongmanee S, Kasetsuwan N, Phusitphoykai N, et al. Clinical evaluation of ophthalmic lomefloxacin 0.3% in comparison with fortified cefazolin and gentamicin ophthalmic solutions in the treatment of presumed bacterial keratitis. J Med Assoc Thai 2004 Sep;87 Suppl 2:S83-90.
5. Diaz Valle D, Alos Cortes JI, Arteaga Sanchez A, et al. Pseudomonas aeruginosa corneal abscess refractory to fluoroquinolones. Arch Soc Esp Oftalmol 2002 Jul;77(7):397-9.
6. Khokhar S, Sindhu N, Mirdha BR. Comparison of topical 0.3% ofloxacin to fortified tobramycin-cefazolin in the therapy of bacterial keratitis. Infection 2000 May-Jun;28(3):149-52.
7. Gangopadhyay N, Daniell M, Weih L, et al. Fluoroquinolone and fortified antibiotics for treating bacterial corneal ulcers. Br J Ophthalmol 2000 Apr;84(4):378-84.
8. Panda A, Ahuja R, Sastry SS. Comparison of topical 0.3% ofloxacin with fortified tobramycin plus cefazolin in the treatment of bacterial keratitis. Eye 1999 Dec;13 ( Pt 6):744-7.
9. Parks DJ, Abrams DA, Sarfarazi FA, et al. Comparison of topical ciprofloxacin to conventional antibiotic therapy in the treatment of ulcerative keratitis. Am J Ophthalmol 1993 Apr 15;115(4):471-7.
10. Hyndiuk RA, Skorich DN,
11. O"Brien TP, Maguire MG, Fink NE, et al. Efficacy of ofloxacin vs cefazolin and tobramycin in the therapy for bacterial keratitis. Report from the Bacterial Keratitis Study Research Group. Arch Ophthalmol 1995 Oct;113(10):1257-65.
12. The Ofloxacin Study Group. Ofloxacin monotherapy for the primary treatment of microbial keratitis: a double-masked, randomized, controlled trial with conventional dual therapy. Ophthalmology 1997 Nov;104(11):1902-9.
13. Hyndiuk RA, Eiferman RA,
14. Sharma V, Sharma S, Garg P, et al. Clinical resistance of Staphylococcus keratitis to ciprofloxacin monotherapy. Indian J Ophthalmol 2004 Dec;52(4):287-92.
15. Rhee MK, Kowalski RP, Romanowski EG, et al. A laboratory evaluation of antibiotic therapy for ciprofloxacin-resistant Pseudomonas aeruginosa. Am J Ophthalmol 2004 Aug;138(2):226-30.
16. Mallari PL, McCarty DJ, Daniell M, et al. Increased incidence of corneal perforation after topical fluoroquinolone treatment for microbial keratitis. Am J Ophthalmol 2001 Jan;131(1):131-3.
17. Hamam RN, Noureddin B, Salti HI, et al. Recalcitrant post-LASIK Mycobacterium chelonae keratitis eradicated after the use of fourth-generation fluoroquinolone. Ophthalmology 2006 Jun;113(6):950-4. Epub 2006 Apr 27.
18. Hyon JY, Joo MJ, Hose S, et al. Comparative efficacy of topical gatifloxacin with ciprofloxacin, amikacin, and clarithromycin in the treatment of experimental Mycobacterium chelonae keratitis. Arch Ophthalmol 2004 Aug;122(8):1166-9.

