Your patient reports that their vision went dark for a couple of seconds in one eye and then went back to normal. What’s your next move? Optometrists are tasked with determining the etiology of sudden, reversible bouts of vision loss. Patients need proper workup due to the wide range of underlying conditions associated with transient monocular vision loss (TMVL). Clinicians must ask critical questions, make careful observations and order appropriate tests to narrow the list of differential diagnoses so that the patient can receive prompt treatment and management (Figure 1).
This article provides a by-the-numbers overview of the information you’ll want to collect, both historical and clinical, how to obtain it and how to interpret it to protect your patients’ vision.
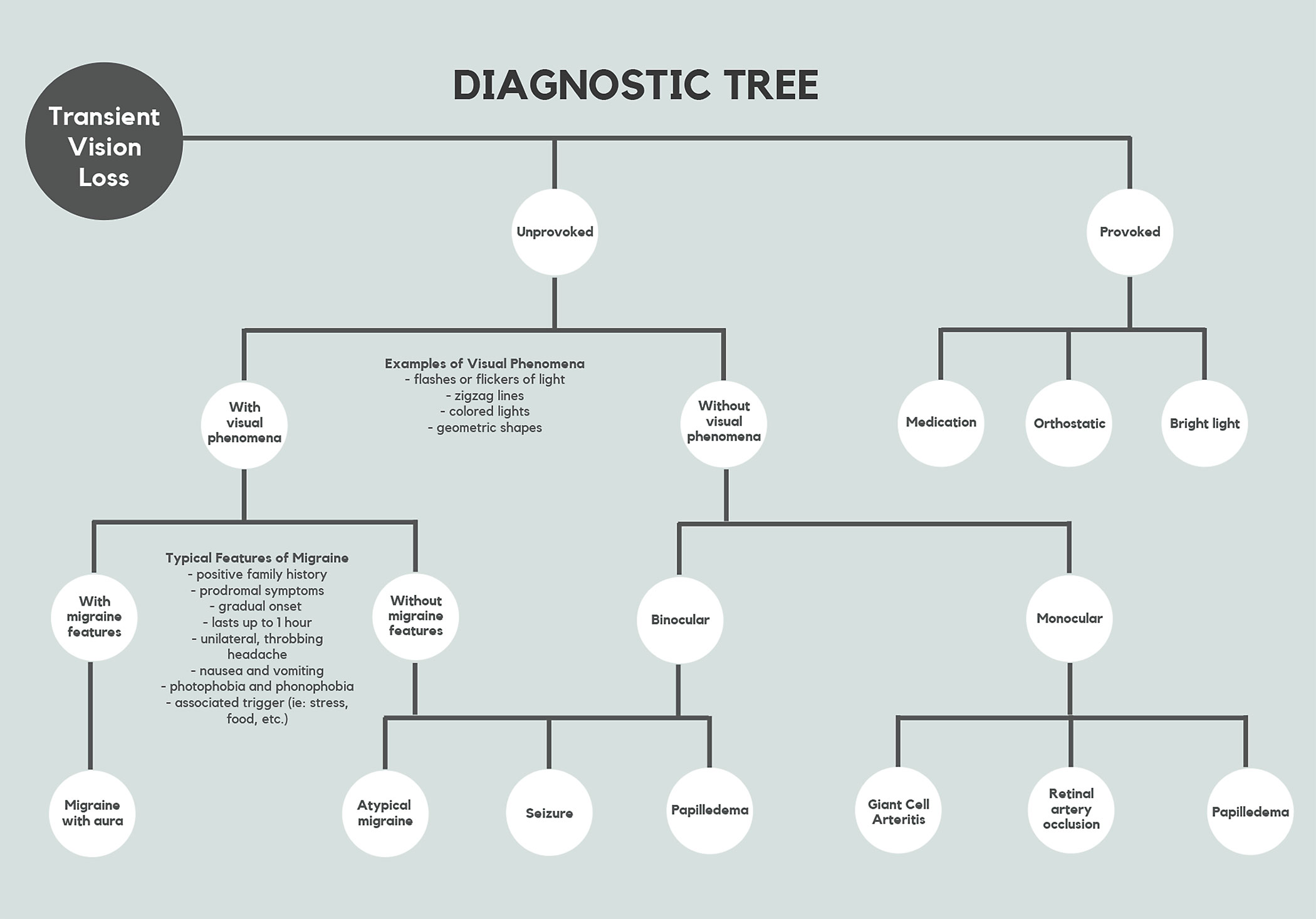 |
| Fig. 1. This flow chart offers a way to navigate TMVL’s various presentations. Click table to enlarge. |
Four Points of Historical Information
Asking about the patients’ experience with these episodes is vital in guiding your next step. The first thing you’ll want to do is get patients to discuss these four aspects of their TMVL.
Monocular/Binocular. The examiner should inquire if the vision loss was in one or both eyes. If it was in one eye, it may be difficult for the patient to accurately recall which eye it was. Transient binocular vision loss (TBVL) can be caused by atypical migraines, papilledema and seizures.1-4 TMVL can be caused by giant cell arteritis (GCA), retinal artery occlusion and thromboembolic events.4 Both binocular and monocular transient vision loss can occur with or without any ocular health abnormalities.1-4 In cases where you observe no ocular pathological findings, it is critical to perform testing such as blood pressure measurement, carotid auscultation and carotid ultrasound to assess the circulatory system.
Duration. Episodes of TMVL can range from a couple of seconds to up to 24 hours.1 It may be difficult for a patient to accurately recall how long the visual disturbance lasted. Regardless, an estimate can help differentiate the cause. TMVL related to migraines tends to last longer and have a gradual onset with a duration of up to one hour.2
Papilledema is a bilateral thromboembolic event that tends to cause quick, one- to two-second bouts of vision loss.1 Thromboembolic-causes of TMVL can last from one to 15 minutes.3
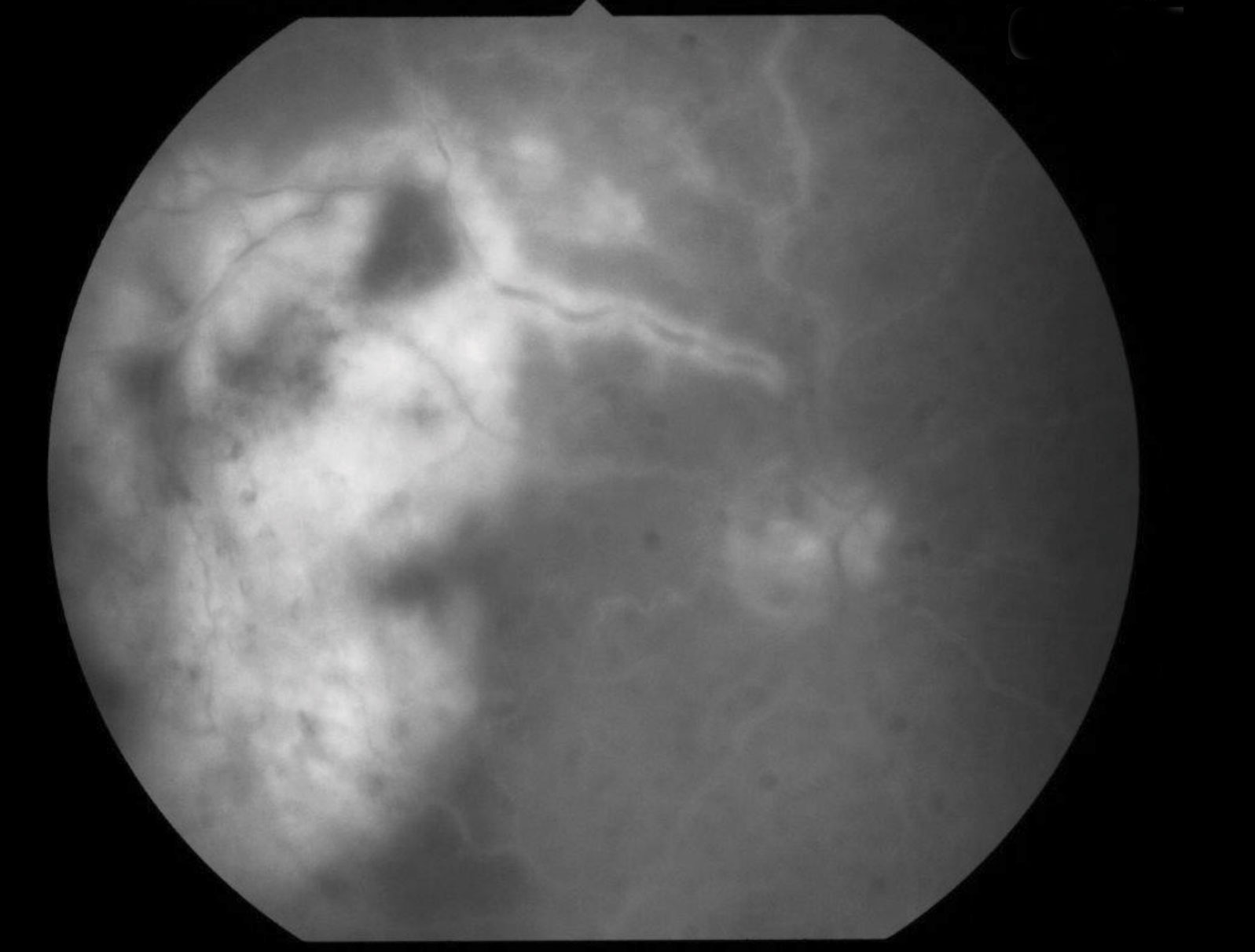 |
| Fig. 3. Fluorescein angiography, seen here, can help identify filling defects of the retinal arteries caused by an occlusion. Click image to enlarge. |
Provoked/Unprovoked. Ask your patient about what they were doing when the vision loss occurred and be sure to verify any recent changes to medications. TMVL can be provoked by a change in the dosage of medications for hypertension.1 Reduced blood perfusion of the optic nerve head during a sudden postural change can also result in brief dimming or loss of vision.1 Delayed recovery following the viewing of a bright light source such as car headlights is another example of provoked TMVL.1
Unprovoked episodes of vision loss are generally more concerning because of the association with thromboembolic events and vascular conditions.
Visual Phenomena. When a patient experiences unprovoked TMVL, the examiner should ask if the patient also experienced brief flashes of light, stationary flickering of light, zigzag lines, colored lights or geometric shapes.
TMVL accompanied by these visual phenomena can be due to a migraine, especially if typical features of a migraine are present.1 Flashing lights can also be a sign of retinal detachment or retinal photopsia caused by vitreoretinal traction. TMVL is a diagnosis of exclusion that requires all organic causes to be ruled out.
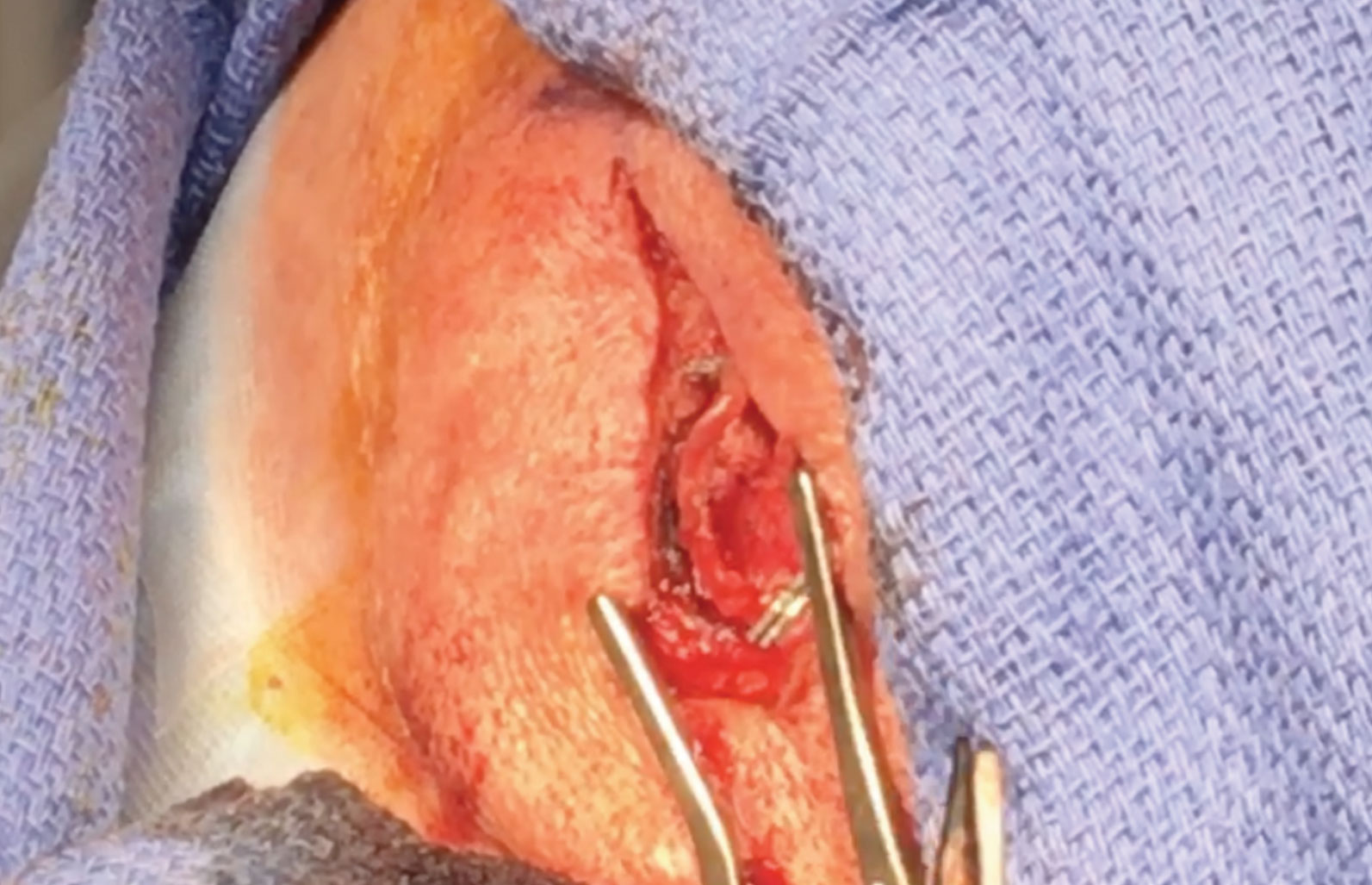
Temporal Artery Biopsy Up CloseThe temporal artery is marked after being located by palpation and Doppler ultrasound. An incision is made through the skin and subcuticular tissue directly over the artery. Dissection is performed with cautery and blunt dissection. The artery is then exposed. It may appear thickened, gray, or mottled compared to a normal artery.6 The specimen to be biopsied is measured. It is recommended that specimens are at least 2cm to 3cm in length due to potential skip lesions.6 The distal and proximal ends of the artery are clamped with titanium clips. The specimen is excised. The incision is closed with dissolvable subcutaneous sutures and the skin with Dermabond. Steri-strips are applied followed by a Telfa pad and pressure dressing. The specimen is sent to pathology to be analyzed for signs of inflammation, including epithelioid cells and giant cells.6 It is rare to have a false-positive temporal artery biopsy.
|
Migraine
The exact pathophysiology of migraines remain unknown, but research suggests that these patients may have overactive neurons in their brains and brainstems resulting in vascular fluctuations, aura and pain.2 The prevalence of migraines with aura is highest in 30- to 50-year-old Caucasian women.2 Research shows that 70% to 90% of patients with migraines have a positive family history.2 Patients with migraines have a higher risk of stroke, especially those who experience aura.2 The distinguishing feature of a migraine from ischemia or seizure is prodromal tingling and numbness, which can start five to 30 minutes before visual aura.1 Headache commonly follows aura and is typically unilateral and throbbing in nature.2 The headaches may be accompanied by nausea, vomiting, photophobia and phonophobia.2
The most common type of aura with migraine is a type of visual phenomena called scintillating scotoma.1 This is often described as sparkling zigzag lines with adjacent blurry, wavy, or missing areas of vision. Other forms of TMVL with migraines are hemianopsias and monocular vision loss.5 In young patients, transient hemianopsias are more often related to migraines than to an embolic transient ischemic attack.5 Transient monocular vision loss due to a migraine will more frequently present with positive visual symptoms in contrast to totally blacked-out monocular vision loss due to an embolus.5
All patients with new migraines and visual aura should have their visual fields assessed. Neuroimaging should be performed as well, especially for patients with a new onset or increased frequency of symptoms. Blood work such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) is critical in elderly patients with TMVL headache symptoms to exclude GCA.2
Since migraine itself is a diagnosis of exclusion, it cannot be identified without appropriate neurologic testing and imaging. If this is suspected, a neurologic referral should be made to pursue the appropriate work up.
Treatment depends on the severity and frequency of the migraines. Non-pharmacologic treatment includes eliminating trigger factors, stress management and biofeedback. Physical techniques such as massage, acupuncture, osteopathic or chiropractic manipulation can be beneficial in certain cases. A number of transcutaneous electrical nerve stimulation devices are now available for migraine treatment. Pharmacologic treatment is divided into acute (abortive) therapy and prophylactic (preventative) therapy. Acute therapies include simple and combination analgesics, non-steroidal anti-inflammatory drugs, steroids, ergot products and selective serotonin receptor agonists known as triptans. Monoclonal antibodies are very target-specific agents that modulate the calcitonin gene-related peptide neurotransmitter.2
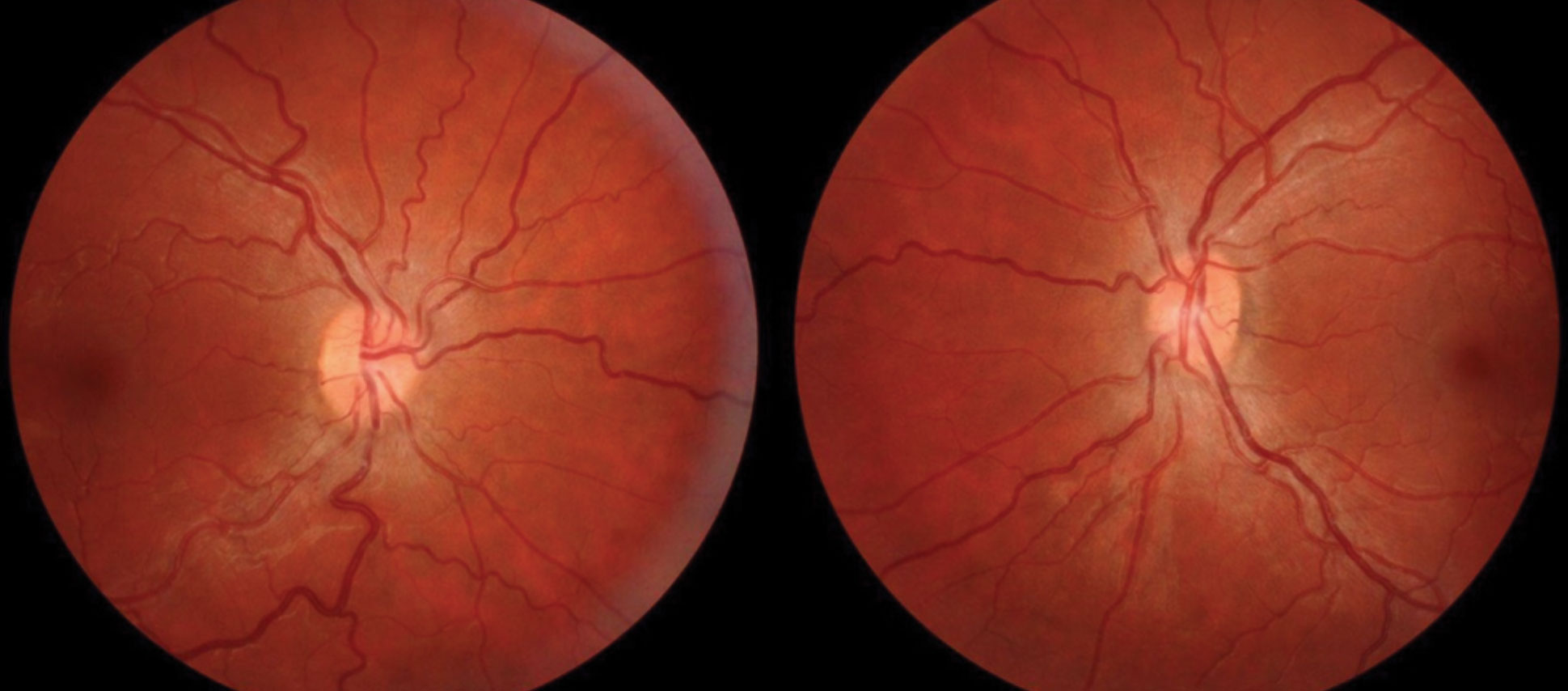 |
| Fig. 4. Conditions that increase the production, or decrease the outflow, of cerebral spinal fluid can lead to papilledema, as seen in this patient’s fundus. Click image to enlarge. |
Giant Cell Arteritis
This inflammatory disease affects medium-to-large vessels with a predilection for arteries of the head, including the ophthalmic artery and superficial temporal artery. Layers of the elastic lamina within the vessels are destroyed by lymphocytes and multinucleated giant cells and inflammation causes narrowing of vessel lumens.6 The prevalence of GCA is three times higher in women than men and most patients are 70 years or older.6 It is uncommon for patients to have GCA if they are younger than 50.6 Research suggests a genetic component may be in play, due to a higher incidence of GCA in Caucasians and an association with HLA antigens.6
Optic nerve infarction tends to manifest as multiple episodes of TMVL before potentially inducing permanent blindness.5 Multiple episodes of TMVL are more likely due to GCA than an embolic cause.5 Transient binocular diplopia and other visual phenomena have been reported in addition to TMVL.6 Approximately 80% to 90% of vision loss in patients who have GCA is due to anterior ischemic optic neuropathy (AION) and the second eye tends to become affected within days in 75% of untreated patients.6
Between 16% and 26% of patients will have visual complications without any systemic symptoms, but the examiner should inquire about headaches, scalp tenderness, jaw claudication when chewing or talking, fever, weight loss and polymyalgia rheumatica.5,6 The most common of these symptoms are headaches and jaw claudication. Interestingly, research shows that patients with jaw claudication may predict a higher risk of vision loss.6
GCA is an ophthalmic emergency whose diagnosis is definitively established with a positive temporal artery biopsy which can be completed by most general ophthalmologists (Figure 2).
The fundus of these patients usually appears normal, but cotton-wool spots may be present, indicating infarction of the retinal nerve fiber layer (RNFL).5 Fluorescein angiography may show choroidal filling defects including delayed filling or patchy defects of perfusion.6
Optic disc edema can result in a chalky, white appearance of the disc with AION. Diagnosis of GCA is highly likely with simultaneous presence of AION and central retinal artery occlusion (CRAO).6
In addition to performing a dilated fundus exam, the examiner should palpate the scalp and jaw muscles to evaluate for any tenderness and a potentially prominent, pulseless temporal artery.6 Blood tests should include erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and complete blood count (CBC) with differential.
An abnormal ESR is usually greater than 90mm/hour, and an abnormal CRP is greater than 2.45mg/dl.6 CRP is a more sensitive test than ESR for diagnosis of GCA although the combination of ESR and CRP is 97% specific for the diagnosis of GCA.6 It is highly unlikely that a patient will have vision loss with a normal ESR, normal CRP and no systemic symptoms. CBC with differential may reveal normochromic normocytic anemia, elevated platelet count or increased inflammatory components in GCA patients.6,7
Patients with GCA are routinely placed on high-dose intravenous corticosteroids to control inflammation and manage symptoms.6 Vision loss due to AION is considered a neuro-ophthalmic emergency that needs immediate intervention to prevent blindness and involvement of the other eye. These patients are started on 1g to 2g of intravenous methylprednisolone for three to five days.6 This is followed by 1mg/kg of oral prednisone per day which will be tapered slowly over six to 12 months.6 ESR and systemic symptoms should improve quickly after the first dose of steroids, but the visual prognosis often remains poor. Symptoms can recur following the initial corticosteroid treatment, vision loss can progress even when on corticosteroid therapy and vision is rarely recovered.6 Recent studies show a reduction in relapse rates with the addition of the steroid-sparing interleukin-6-receptor antagonist tocilizumab.6,8
Case Report: Patient With Transient Vision LossAn 82-year-old man presented to the eye clinic with a concern about recent episodes of transient vision loss. The patient explained that vision in his right eye would go completely black for about half an hour, and then his vision would recover after lying down to rest. The patient also reported a history of vertical diplopia that had occurred sporadically in the past few months. The patient denied any visual phenomena or pain related to the vision loss, but he did report jaw discomfort that occurred occasionally while eating. Review of systems was significant for sarcoidosis, for which the patient was currently taking 25mg of prednisone. The patient reported that his family physician recently tapered the prednisone from 30mg to 25mg, and this coincided with the occurrence of his transient vision loss. No evidence of any ocular involvement was found upon examination. Blood tests were ordered by his family physician, and the results showed elevated ESR, elevated CRP and slightly elevated platelet, neutrophil and monocyte counts. We performed a temporal artery biopsy on the right side since visual symptoms were in his right eye. The results came back positive for giant cell arteritis (Figure 5). As a result, the dosage of prednisone was increased to 40mg per day by his family physician to manage the patient’s symptoms and prevent vision loss. |
Retinal Artery Occlusion
Temporary interruption of retinal circulation can cause TMVL, also known as amaurosis fugax.5 Interruptions are usually due to cholesterol, platelet-fibrin or calcific emboli.9 Blockages can result in a central retinal artery occlusion (CRAO) or a branch retinal artery occlusion (BRAO), which can lead to permanent severe vision loss.5,10 One study shows that more than 40% of patients with a CRAO had a plaque located in their internal carotid artery near where the ophthalmic artery originates.5 Retinal artery occlusions are most common in males and patients in their 60s.10 Risk factors for retinal artery occlusion include carotid disease and cardiac disease.1 Diabetes, hypertension, high cholesterol, certain blood conditions and smoking are also risk factors.5,10 Artery occlusion today is considered an active ‘stroke in the eye’ and requires immediate admission to the hospital with evaluation from a certified stroke team.
Vision loss caused by a retinal artery occlusion typically has an abrupt painless onset.5 The episode generally lasts for one to 15 minutes until the blockage disperses and blood flow is restored.5 The episode of TMVL is usually described as darkening rather than blurring of vision. If vision returns it does so slowly over minutes.5 Only about 1% to 2% of cases affect both eyes.10 If the acuity returns the patient may realize they have developed permanent visual field defects that can be altitudinal, peripheral or central.5 Nasal visual field defects are common because emboli tend to get trapped in the temporal retinal circulation.5
With CRAO, the fundus will have a “cherry red spot” at the center of the macula, and the rest of the retina will appear pale due to the lack of blood supply.5,10
With BRAO, you’ll see an area of pale retina that corresponds to the blocked vessel (Figure 3).10 Paleness of the retina tends to last around four to six weeks before fading away as the tissue atrophies.10 Fluorescein angiography can identify any filling defects of the retinal arteries. In addition, a measurement of the patient’s blood pressure should be obtained. For carotid emboli, the carotid artery can be assessed with conventional angiography, a Doppler ultrasound, magnetic resonance angiography or computer tomography angiography.5 Cardiac evaluation including electrocardiography and 24-hour heart monitoring is warranted in cases of cardiac emboli.5 Neuroimaging of the head may also be indicated to look for any emboli in the brain.5
There is no standard treatment for retinal artery occlusions. Suggested therapies attempt to dislodge the embolus with ocular massage, hyperventilation by breathing into a paper bag, which may cause dilation of the vessels, and lowering intraocular pressure with paracentesis or medication.10 These treatments must be implemented within four to six hours after the onset of symptoms to be effective.10 A recent study found that intravenous PGE1 infusion, a prostaglandin infusion therapy, could statistically significantly recover vision in patients with acute CRAO.11 Management of a patient with retinal artery occlusion is focused on reducing the risk of stroke and other vascular events. Medications include statins and antiplatelet therapy with aspirin.5 In addition, patients are advised to control any underlying hypertension and blood glucose levels, quit smoking and lose weight to reduce the risk of another retinal artery occlusion or a future stroke.5
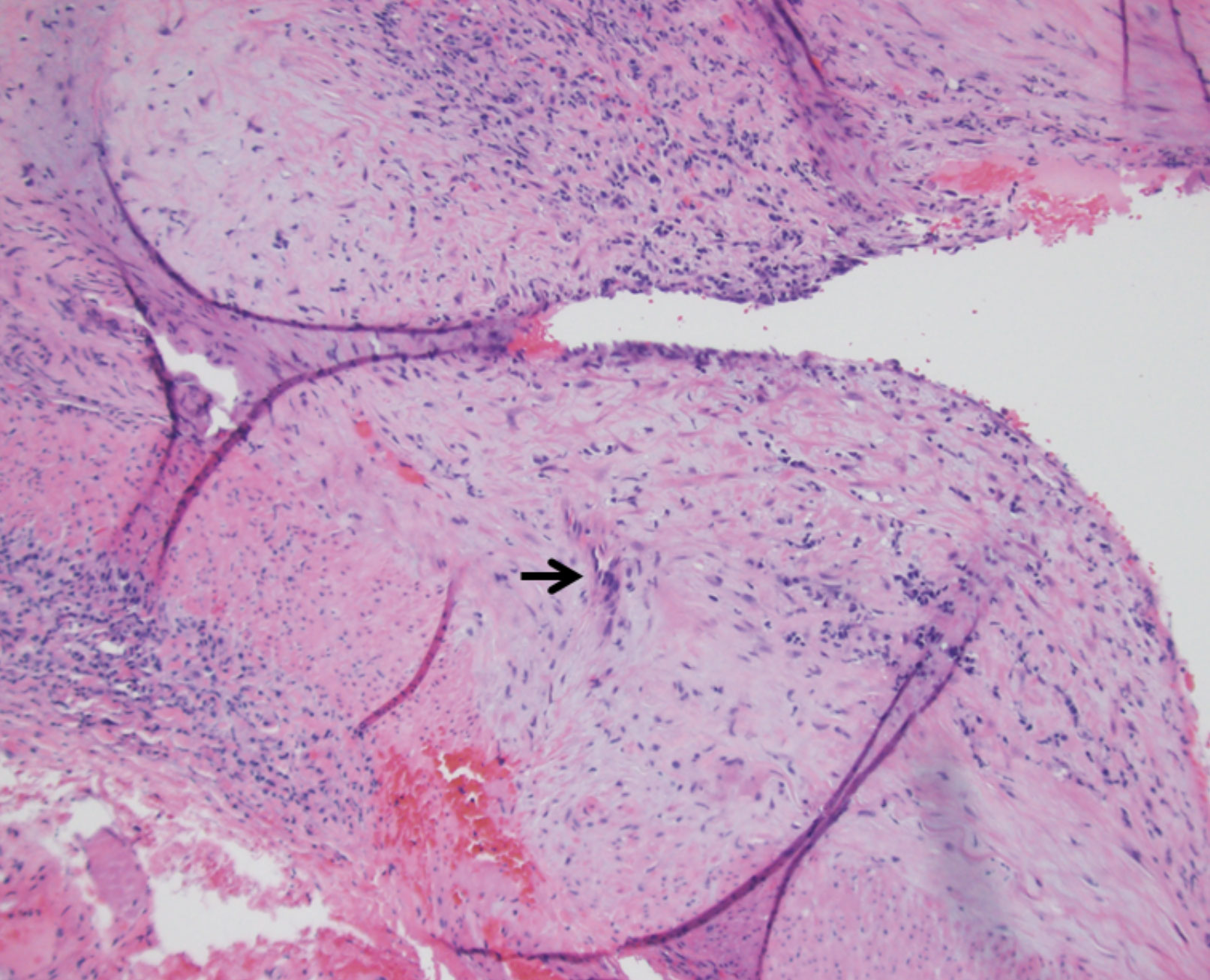 |
| Fig. 5. Histology shows a specimen affected by temporal arteritis (the arrow indicates a multinucleated giant cell). Click image to enlarge. |
Papilledema
This condition is characterized by swollen optic discs which occur due to increased intracranial pressure (ICP). This ICP increase can disrupt axoplasmic flow through the optic nerve and result in the leakage of cellular contents around the optic disc (Figure 4).12
Papilledema may result from pathologies such as intracranial mass, choroid plexus papilloma, obstructive hydrocephalus, hemorrhagic cerebrovascual accident and venous sinus thrombosis. It may also occur idiopathically as in idiopathic intracranial hypertension (IIH).
IIH mostly affects overweight females of child-bearing age between 15 and 44 years old, but it can affect all ages and genders.13 No proven cause is known, hence the idiopathic etiology of the condition. The proposed pathophysiology is a combination of a decreased absorption or increased production of CSF related to vascular, hormonal, or cellular mechanisms.13
TMVL is not usually the presenting symptom in patients with papilledema, but it is a common symptom, occurring in up to 70% of IIH cases.13 Vision loss can be in one or both eyes and can cause a partial or complete visual field defect.
Vision changes are often described as a monocular or bilateral “graying out,” with episodes usually only lasting a couple seconds.14 Patients with papilledema may also experience binocular horizontal diplopia due to a lateral rectus (CN VI) palsy.13 Visual phenomena such as flashes of light have been reported in 54% of cases and permanent vision loss in 32% of untreated cases.13 Chronic, untreated papilledema can lead to field defects that mimic the damage caused by glaucoma.15
Headaches occur in 98% of cases of papilledema.13 They can be in any location and occur daily. Headaches may be accompanied by nausea, vomiting, light sensitivity, pulsatile tinnitus, and neck and back pain.13 The headache with IIH is usually worse in morning and may have a positional component.
The evaluation of a patient who presents with signs and symptoms of IIH includes dilated fundus examination, visual field assessment, neuroimaging, lumbar puncture, CSF analysis and CBC, best accomplished by the neurologist or neuro-ophthalmologist. In-office assessment with an optical coherence tomographer can demonstrate a swollen nerve fiber layer.
The severity of papilledema seen on dilated fundus examination may correspond with the severity of vision loss.13 An assessment of the patient’s visual field will likely reveal an enlarged blind spot or inferonasal field loss.13 Magnetic resonance imaging (MRI) with venography (MRV) can help rule out various other causes of increased ICP such as dural venous sinus thrombosis. If MRI is contraindicated, computed tomography (CT) can be done instead. Abnormal opening pressures greater than 25cm H2O in adults and 28cm H2O in children up to age 18 may suggest a diagnosis of IIH.13 An analysis of CSF should be conducted as well as a CBC to further rule out other causes of intracranial hypertension. Diagnosis of IIH is ultimately established using the Modified Dandy Criteria.13
Lumbar puncture can temporarily relieve symptoms or completely resolve papilledema in a patient with IIH.13 Patients should be advised to lose 5% to 10% of body weight and modify their diets to increase the chances of remission.13 Pharmacologic agents such as acetazolamide and furosemide aim to decrease the production of CSF and may have a diuretic component for increasing outflow of CSF. Topiramate can be prescribed for migraine prophylaxis, and it also has a carbonic anhydrase inhibitor component that can help the patient with weight loss.13
Steroids can help quickly lower ICP as well but can lead to rebound weight gain and ICP increase when taken off the steroids.13 For these reasons, steroids should only be prescribed in severe cases of vision loss and in cases that do not respond to other medications.
Surgery is another option for certain refractory cases. In optic nerve sheath decompression, slits are made in the optic nerve sheath to increase the outflow of CSF and decrease pressure exerted on optic nerve.13 A different surgery called CSF diversion uses a shunt and is more effective at reducing headaches than vision loss.13 It may take months to years to treat this condition. Patients may still have papilledema, increased ICP, and visual field defects even with treatment.
Transient vision loss is a common visual complaint of patients, and, in certain cases, a prompt diagnosis and initiation of treatment and management could be critical to not only the patient’s vision but their overall health. There are many factors to consider when managing such a patient and optometrists need to be ready to do so efficiently. Optometrists should know what information and tests are pertinent for a diagnosis and then decide whether the patient should make a visit to another medical specialist or the emergency room for the care that the patient needs.
Dr. Tran is a fourth-year optometry student at Pacific University. She expects to graduate in May 2020.
Dr. Skorin practices at the Mayo Clinic Health System in Albert Lea, Minnesota.
1. Burde RM, Savino PJ, Trobe JD. Transient vision loss. Clinical Decisions in Neuro-Ophthalmology. 2002;3:94-111. 2. Ko M, Prasad S. Headache, facial pain and disorders of facial sensation. In: Liu, Volpe, and Galetta, ed. Liu, Volpe, and Galetta’s Neuro-Ophthalmology. 3rd ed. Philadelphia, PA: Elsiver; 2019:661-684. 3. Donders R. Clinical features of transient monocular blindness and the likelihood of atherosclerotic lesions of the internal carotid artery. J Neurol Neurosurg Psychiatry. 2001;71(2):247-9. 4. Pula J, Kwan K, Yuen C, et al. Update on the evaluation of transient vision loss. Clin Ophthalmol. 2016;10:297-303. 5. Tamhankar MA. Transient Vision Loss or Blurring. In: Liu, Volpe, and Galetta, ed. Liu, Volpe, and Galetta’s Neuro-Ophthalmology. 3rd ed. Philadelphia, PA: Elsiver; 2019:365-377. 6. Pineles SL, Balcer LJ. Visual Loss: Optic Neuropathies. In: Liu, Volpe, and Galetta, ed. Liu, Volpe, and Galetta’s Neuro-Ophthalmology. 3rd ed. Philadelphia, PA: Elsiver; 2019:101-196. 7. Walvick MD, Walvick MP. Giant cell arteritis: laboratory predictors of a positive temporal artery biopsy. Ophthalmol. 2011;118(6):1201-4. 8. Stone J, Tuckwell K, Dimonaco M, et al. Trial of tocilizumab in giant cell arteritis. N Engl J Med. 2017;377(7):317-328. 9. Miller NR. Embolic causes of transient monocular vision loss: Appearance, source, and assessment. Ophthalmol Clin North Am. 1996;9(3):359-80. 10. Bakri S, Capone A, Ciulla T, et al. Retinal artery occlusion. Am Soc Retina Specialists. www.asrs.org/content/documents/fact-sheet-29-retinal-artery-occlusion.pdf. 2017. Accessed July 17, 2019. 11. Malbin B, Padidam S, Burke M, et al. Intravenous prostaglandin E1 infusion for acute central retinal artery occlusion. Ophthalmic Surg Lasers Imaging Retina. 2019;50(5):S5-S8. 12. Minckler DS, Tso MO, Zimmerman LE. A light microscopic, autoradiographic study of axoplasmic transport in the optic nerve head during ocular hypotony, increased intraocular pressure, and papilledema. Am J Ophthalmol. 1976;82(5):741-57. 13. Mondragon J, Klovenski V. Pseudotumor cerebri. StatPearls. www.ncbi.nlm.nih.gov/books/NBK536924/. January 26, 2019. Accessed July 17, 2019. 14. Sadun A, Currie J, Lessell S. Transient visual obscurations with elevated optic discs. Ann Neurol. 1984;16(4):489-94. 15. Pearson P, Baker R, Khorram D, et al. Evaluation of optic nerve sheath fenestration in pseudotumor cerebri using automated perimetry. Ophthalmology. 1991;98(1):99-105. |