Mind-Eye ConnectionThe October 2024 issue of Review of Optometry focuses on the treatment and management of neuro-ophthalmic disorders. Check out the other articles featured in this issue: |
The correspondence of the eye and the central nervous system (CNS) is one that is well-established and of particular interest in the arena of neurodegenerative diseases (NDDs). NDDs are onerous to diagnose in their initial stages, and once identified, hold guarded prognosis since treatments are palliative. As the understanding of NDD mechanisms rapidly evolves and improved treatments targeting these disease processes have been developed, the eye has become a focal point of what may soon provide a roadmap for early NDD detection. To better appreciate this significance, one must first understand the difficulty NDDs present to our patients and our health system.
Whether it be Alzheimer’s, multiple sclerosis, Parkinson’s or frontotemporal dementia, all NDDs are characterized by nerve cells in the brain and elsewhere losing function and ultimately dying.
The impact NDDs have on the health system is staggering: worldwide, over 15% of the population is affected by NDDs which cause physical and cognitive disability, and in America, it is estimated that over 10 million people suffer from these conditions.1,2 The financial implications are also eye-opening, with some studies estimating $800 billion annually is currently spent on diagnosis, treatment and management of these diseases.3 The most concerning aspect of this statistic is that NDDs are on track to be the most expensive disease state, with numbers that are growing and could potentially triple by 2050.4
The NDD Model
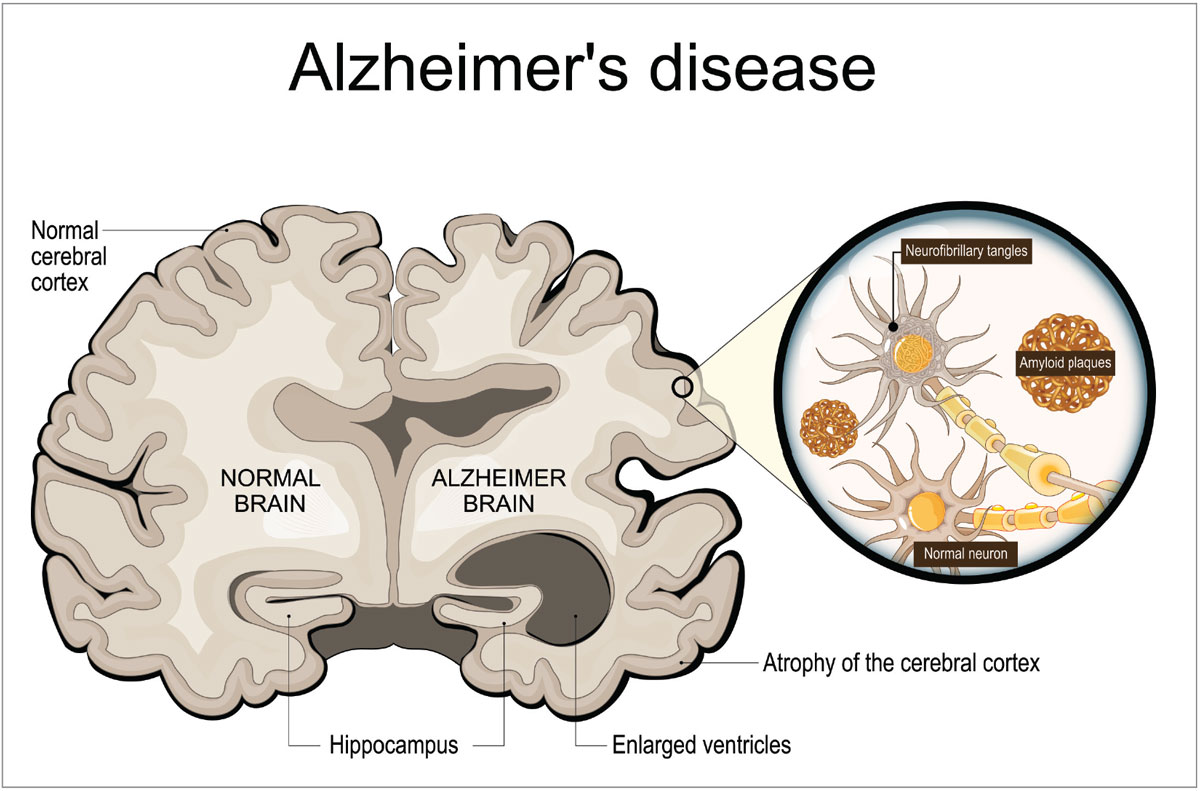
Alzheimer’s disease (AD) accounts for over 60% of all NDD cases and serves as a critical case study in understanding the impact of these diseases more generally.2 AD causes cognitive decline and typically affects patients older than 65, having a slightly higher prevalence in women.5 AD is a dual proteinopathy characterized by the deposition of amyloid beta and tau proteins, which interfere in the normal function of neurons located in the cerebral cortex (Figure 1). In a healthy neuron, amyloid beta serves as a shield to stressors and enhances synaptic function, while tau proteins help to maintain neuron structure by stabilizing microtubules.6 As Alzheimer’s develops, amyloid beta forms into plaques that effectively block neurotransmitters at the acetylcholine junction, and neurofibrillary tangles of tau proteins lead to destabilization of microtubules in the axon of the neuron, leading to breakdown in conduction.7 Years before the onset of even early cognitive impairment, these cellular changes have been identified in the brains of affected individuals.8
 |
|
Fig. 1. Neurofibrillary tangles and amyloid plaques accumulate and disrupt neuronal function, resulting in atrophy of the cerebral cortex. Photo: Getty Images. Click image to enlarge. |
As the disease progresses, neuroimaging often demonstrates atrophy throughout the temporoparietal cortex, leading to mild, moderate and then advanced cognitive and functional decline.5 Clinical signs of Alzheimer’s may be memory loss, sleep cycle disruption, executive function loss, mood changes and spatial relation difficulty. In the advanced stages of the disease, patients most often succumb to aspiration pneumonia after motor function loss leads to dysphagia.9 Early diagnosis and initiation of improved treatments can enhance and extend these patients’ lives, but due to the insidious nature of the disease, detecting its onset at an early stage is often laborious and expensive or just plain overlooked.
Although most NDDs exhibit varying patterns in prevalence, risk factors, symptoms and clinical manifestations (Table 1), they, like Alzheimer’s, all present significant diagnostic challenges and offer limited efficacy with current treatments. To identify patients with NDDs at earlier stages, biomarkers have been propelled to the forefront in hopes of improving patient outcomes and thereby eliminating financial strain on the health system. The eye itself presents a microcosm of biomarkers that may hold the key to better identifying these patients and offering treatments at an earlier stage.10 This poses the question, “What if an eye exam could uncover the connection with a NDD in an undiagnosed patient?”
 |
| Click image to enlarge. |
The Brain-Eye Connection
Since the brain, spinal cord, retina and optic nerve all derive from the same embryonic cells, diseases affecting the CNS may potentially impact neurons in the eye. Studying alterations to the neurons and vasculature in the eye provides a window into changes occurring in the brain.
In patients with NDDs, the neuronal atrophy and pathophysiological changes seen in the brain are mirrored in the retina. For instance, in patients with AD, amyloid beta and phosphorylated tau protein deposits are found in the inner retina.11,12 Research indicates that amyloid beta deposits are more prevalent in the superior temporal quadrant of the retina. These deposits are thought to be linked to the thinning of the retinal nerve fiber layers and ganglion cells in this region, as observed through OCT imaging.13 In patients with Parkinson’s disease (PD), laboratory studies have revealed a reduction in the neurotransmitter dopamine, which can impact the functionality of retinal ganglion cells (RGCs) and the retinal pigment epithelium.14 Due to similar pathophysiological mechanisms, the total overall neuronal atrophy occurring in the brain of NDD patients is also occurring in the neurons in the retina.15 Noninvasive retinal imaging techniques, such as peripapillary and ganglion cell layer/inner plexiform layer OCT scans, have heightened interest in the eye as a potential site for early biomarkers of these conditions.
Further evidence of the link between the eye and NDDs comes from studies of melanopsin-containing RGCs (mRGCs), which are intrinsically photosensitive RGCs (ipRGCs) that express melanopsin. Unlike most other RGCs, which transmit image-forming light messages to the visual pathway, ipRGCs are integral in non-image forming functions, such as regulating the pupillary light reflex and circadian rhythms; they also project to brain areas involved in regulating mood and cognitive function. In patients with NDDs, mRGCs often show an abnormal number and structure when compared with age-matched controls.16 The disrupted function of mRGCs is the likely cause of common characteristics of NDDs, which include altered pupillary light responses, circadian disruptions, cognitive decline and mood disorders. This makes ipRGCs promising candidates for early diagnostic tools.17 One can also consider that if there was a way to preserve the function of ipRGCs, perhaps this could potentially alleviate some symptoms of NDDs, thereby enhancing a patient’s quality of life.
Although large-scale epidemiological studies have yielded mixed results, increasing evidence suggests that eye disorders are associated with the presence of AD, dementia and other NDDs.18,19 While NDDs are often thought of as diseases affecting the brain, some common eye diseases also exhibit neurodegenerative features. For example, ipRGCs are depleted in NDDs, and they are also depleted in eye disorders such as glaucoma, diabetic retinopathy, macular degeneration and inflammatory and autoimmune conditions. Additionally, certain types of glaucoma share genetic features similar to Alzheimer’s and Parkinson’s, and retinal amyloid beta’s role in macular degeneration overlaps with pathology in AD.20,21
The connection between eye disorders and NDDs is partly driven by common neuroinflammatory cascades. In both the CNS and eye, protein aggregates trigger glial cell and astrocyte activation, leading to neuroinflammation and subsequent neurodegeneration. Contributing factors to neuroinflammation include genetic predispositions, environmental influences, infections, nutrition and lifestyle choices.22 Addressing risk factors which are modifiable may offer promising targets for intervention in high- risk populations.
The Eyecare Provider’s Role
So, how can an optometrist make an impact on patients with a diagnosis or those who may not know they have an NDD? A thorough history is the first foray into the evaluation of a potential NDD patient and is critical for knowing when to dig deeper. Based on the patient profile or level of concern from a caretaker, further questioning and testing may be indicated. For instance, there is no clinical test readily available to evaluate spatial relations, but a history reasonable for an eye doctor to inquire about may include probing mobility issues at home, bumping into objects, recent falls, depth perception and nighttime awareness while walking within the home.23 Navigation while driving may be an area to explore in association with distance vision, too. Memory is also an issue we don’t typically assess with our exams; however, reading comprehension, loss of concentration and visual recognition of familiar faces, places and objects are all areas to probe and investigate that align with an eye professional’s expertise.24 In summary, consider adding additional history attention to areas such as memory, mobility, navigation and spatial awareness for our older patients or anyone who is diagnosed with an NDD.
Testing. Capturing relevant information in an exam can help connect the dots for those patients who may be diagnosed or undiagnosed with an NDD. Clinical manifestations can be subtle and varied with the myriad of NDDs and their impact on the visual system. Additional testing may include contrast sensitivity and color vision when available.25,26 Patients with indeterminate visual complaints should undergo visual field testing, as multiple NDDs have been associated with defects.27 This is especially true in those with suspected Alzheimer’s. Patients with Alzheimer’s tend to express greater thinning in the superior retinal nerve fiber layer, resulting in inferior field defects. There is also a visual variant of AD known as Benson’s syndrome or posterior cortical atrophy that commonly presents with a homonymous visual field defect.28 Electroretinography is a technology that is now readily available and integrated in diabetic and glaucoma care that could also be considered in baseline for patients diagnosed or suspected of an NDD.29 Patients with NDDs may show an increase in implicit time and/or a decrease in amplitude.
The most promising and tangible technology that optometry has become accustomed to using in patient care is OCT. As defined previously, multiple biomarkers have been identified for many NDDs, and a baseline OCT could be considered for patients older than 65 to help monitor for changes over time.30-32 An abnormal OCT in a patient who fits the profile for an NDD should be weighed with other possible contributing eye diseases rather than it serving as a definitive indicator of NDD. A helpful way to consider these patients is to prioritize ocular history and other risks that fall outside the glaucoma spectrum of optic neuropathy. No one test or ocular biomarker is currently diagnostic for NDDs, but studies are improving. Optometry should be on the front lines in knowing the relevant testing that can solve the puzzle of treating these patients—and to help lead them to the proper care when a diagnosis is suspected.33
Comorbidities. Once a patient has been diagnosed with an NDD, careful consideration should be given to daily function, the ocular surface and ocular comorbidities. Visual tasks like reading can be unburdened by detailed refraction and consideration of tints and filters specific to patient symptoms.
Address symptoms such as photophobia, reduced contrast and diplopia attentively, as many patients with cognitive decline will have difficulty characterizing their symptoms.34,35 Indoor filters such as rose-colored FL-41 can reduce photophobia or migraine phenomenon, yellow filters may improve contrast and a thorough ocular motility exam can help alignment issues. If you do not feel that you can fully address your patient’s needs in your office, don’t forget that our low vision colleagues specialize in devoting the attention needed to improve visual function with devices, filters and reading aids.
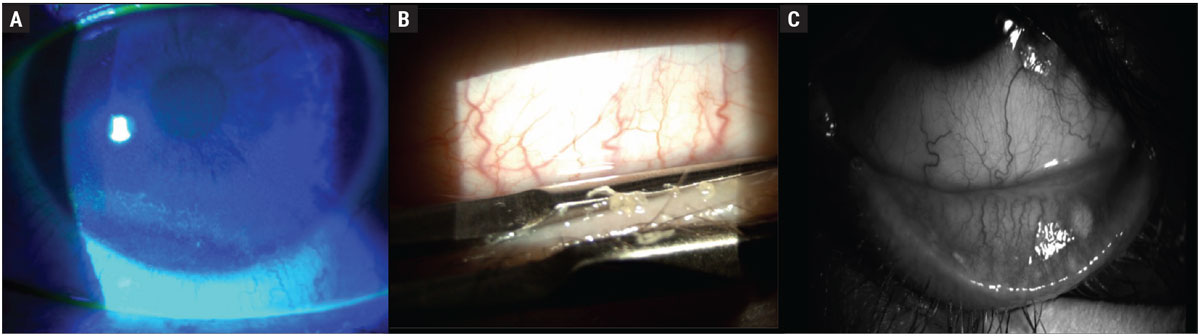
Optimizing the ocular surface should not be overlooked in this patient subset. Multiple studies have shown that sensory neurotrophic effects can also impact the corneal surface and tear film (Figure 2).36-38 Taking the time to implement a treatment that doesn’t add a cumbersome daily commitment can pay dividends for patients with poor dexterity or memory issues. These could include treatments such as in-office heat meibomian gland treatments, varenicline nasal spray or punctal plugs.
 |
|
Fig. 2. Examples of corneal surface and tear film effects of neurodegenerative diseases: (A) corneal epithelial erosions, which stain positive with fluorescein, (B) inspissated meibomian glands, which can undergo in-office expression, (C) atrophy and dropout of meibomian glands. Click image to enlarge. |
Addressing comorbidities that affect NDD patients is paramount and may include cataracts, glaucoma or macular degeneration.39,40 Cataracts are of particular interest and, upon removal, have been shown to improve cognitive function and reduce the risk for dementia by up to 30%.41,42 This may be linked to the level of blue light prohibited from interacting with ipRGCs due to the yellowing of the crystalline lens—offering yet another reason to refer for surgery when a patient may be reluctant to undergo an operation.
Lifestyle. One area that ODs tend to excel at is speaking to lifestyle considerations. Whether it be for our dry eye or diabetes patients, diet, exercise and supplements are one area that optometry takes a leading role in for discussing proactive interventions. Mediterranean style diets and derivatives of this diet have been shown to slow neurodegenerative disease and improve cognitive function.43 Even caloric restriction alone has been shown to improve brain health and cognitive function.44 Exercise also shows promise as an NDD-modifying therapeutic approach, with moderate activity for 45 minutes, three to five times weekly showing benefits.45
Supplementation. Nutraceutical supplements remain a highly debated topic in healthcare. Nevertheless, in the context of NDDs, substantial evidence supports their benefits—particularly if a patient is deficient. Three supplements worth mentioning that are well-documented to show benefits are curcumin, vitamin D and omega-3.
Curcumin is of particular interest due to its antioxidant, anti-inflammatory and immunomodulatory properties. It has demonstrated supplemental benefits when used alongside medical therapy, particularly in patients with neuroinflammation, such as those with MS.46 Vitamin D, which gained notoriety during the COVID-19 pandemic, is essential to measure, as deficiencies are common in individuals with NDDs. Proper supplementation of vitamin D has shown significant potential for curbing NDD progression. A 2023 study looked at 10,000 patients over a 10-year period and demonstrated a 40% reduction in cognitive decline in those who supplemented with vitamin D.47
Omega-3 fatty acids, commonly recommended for dry eye patients, also offer substantial benefits for NDDs. Docosahexaenoic acid, the primary active fatty acid in the brain, has been highlighted in recent research from the UK Biobank for its positive effects on neurodegenerative conditions.48 In summary, looking at the big “lifestyle” picture, including appropriate supplementation, can be a valuable strategy for preventing disease onset or as a supplement to medical therapy for patients who are treated for NDDs. This approach offers optometrists another way to contribute to the overall improvement of patient health.
Takeaways
The intricate relationship between the eye and CNS offers a promising frontier in the early detection and management of NDDs. As we navigate the growing challenges posed by conditions like Alzheimer’s, Parkinson’s and other NDDs, the eye emerges as a critical tool for uncovering early biomarkers and potentially transforming diagnosis and treatment strategies. Given the shared neuroanatomical and pathological features between the eye and CNS, noninvasive technologies like OCT and advanced retinal imaging provide invaluable insights into the neurodegenerative processes at play. Neuronal atrophy of the retina may be able to be observed earlier than brain neuronal atrophy, which may allow for earlier diagnosis and treatment of NDDs.
The role of optometrists extends beyond traditional vision care; we are well-positioned to detect subtle signs of cognitive decline and provide comprehensive assessments that integrate visual function with neurological health. By incorporating targeted screening for NDDs, addressing visual and ocular comorbidities and promoting lifestyle modifications, optometrists can significantly impact patient outcomes and contribute to a more holistic approach to managing neurodegenerative conditions. As research continues to unravel the complexities of these diseases, the synergy between ophthalmic and neurological care will be crucial in advancing early detection, improving quality of life and ultimately alleviating the substantial burden these diseases impose on individuals and healthcare systems alike.
Dr. Draper is an associate professor at the Pennsylvania College of Optometry at Salus University. She provides clinical care at The Eye Institute in Philadelphia in the Neuro-Ophthalmic Disease Service. She also serves on the Pennsylvania Optometric Association Board of Directors and is a fellow of the American Academy of Optometry. She has no financial disclosures.
Dr. Kuc is the medical director at Medical Optometry America. With over 20 years of clinical experience in ocular surface disease, glaucoma, emergency eye care and surgical comanagement, he brings his proactive- and prevention-based approach to eye care to this suburban Philadelphia community. He is a Fellow of the American Academy of Optometry and a Diplomate of the American Board of Optometry and currently serves on the board for the Pennsylvania Optometric Association. He is a consultant for Zeiss and Tarsus.
1. Van Schependom J, D’haeseleer M. Advances in neurodegenerative diseases. J Clin Med. 2023;12(5):1709. 2. Thorpe KE, Levey AI, Thomas J. U.S. burden of neurodegenerative disease: literature review summary. Partnership to Fight Chronic Disease. fightchronicdisease.org/sites/default/files/may%202021%20neurodegenerative%20disease%20burden%20on%20us%20-%20final%20.pdf. 2021. Accessed July 12, 2024. 3. The burden of brain disease. American Brain Foundation. www.americanbrainfoundation.org/the-burden-of-brain-disease. May 9, 2023. Accessed July 12, 2024. 4. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778-83. 5. 2024 Alzheimer’s disease facts and figures. Alzheimer’s Association. www.alz.org/media/documents/alzheimers-facts-and-figures.pdf. Accessed July 14, 2024. 6. Kent SA, Spires-Jones TL, Durrant CS. The physiological roles of tau and Aβ: implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol. 2020;140(4):417-47. 7. Atri A. The Alzheimer’s disease clinical spectrum: diagnosis and management. Med Clin North Am. 2019;103(2):263-93. 8. Shafiee N, Fonov V, Dadar M, Spreng RN, Collins DL. Degeneration in nucleus basalis of Meynert signals earliest stage of Alzheimer’s disease progression. Neurobiol Aging. 2024;139:54-63. 9. Kalia M. Dysphagia and aspiration pneumonia in patients with Alzheimer’s disease. Metabolism. 2003;52(10 Suppl 2):36-8. 10. Patterson EJ, Bounds AD, Wagner SK, et al. Oculomics: a crusade against the four horsemen of chronic disease. Ophthalmol Ther. 2024;13(6):1427-51. 11. den Haan J, Morrema THJ, Verbraak FD, et al. Amyloid-beta and phosphorylated tau in post-mortem Alzheimer’s disease retinas. Acta Neuropathol Commun. 2018;6(1):147. 12. Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54 Suppl 1:S204-17. 13. Wang L, Mao X. Role of retinal amyloid-β in neurodegenerative diseases: overlapping mechanisms and emerging clinical applications. Int J Mol Sci. 2021;22(5):2360. 14. Alves JN, Westner BU, Højlund A, Weil RS, Dalal SS. Structural and functional changes in the retina in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2023;94(6):448-56. 15. Doustar J, Torbati T, Black KL, Koronyo Y, Koronyo-Hamaoui M. Optical coherence tomography in Alzheimer’s disease and other neurodegenerative diseases. Front Neurol. 2017;8:701. 16. Lax P, Ortuño-Lizarán I, Maneu V, Vidal-Sanz M, Cuenca N. Photosensitive melanopsin-containing retinal ganglion cells in health and disease: implications for circadian rhythms. Int J Mol Sci. 2019;20(13):3164. 17. Matynia A, Recio BS, Myers Z, et al. Preservation of intrinsically photosensitive retinal ganglion cells (ipRGCs) in late adult mice: implications as a potential biomarker for early onset ocular degenerative diseases. Invest Ophthalmol Vis Sci. 2024;65(1):28. 18. Zheng C, Zeng R, Wu G, Hu Y, Yu H. Beyond vision: a view from eye to Alzheimer’s disease and dementia. J Prev Alzheimers Dis. 2024;11(2):469-83. 19. Feng J, Huang C, Liang L, et al. The association between eye disease and incidence of dementia: systematic review and meta-analysis. J Am Med Dir Assoc. 2023;24(9):1363-73.e6. 20. Artero-Castro A, Rodriguez-Jimenez FJ, Jendelova P, et al. Glaucoma as a neurodegenerative disease caused by intrinsic vulnerability factors. Prog Neurobiol. 2020;193:101817. 21. Ashok A, Singh N, Chaudhary S, et al. Retinal degeneration and Alzheimer’s disease: an evolving link. Int J Mol Sci. 2020;21(19):7290. 22. Tanaka M, Toldi J, Vécsei L. Exploring the etiological links behind neurodegenerative diseases: inflammatory cytokines and bioactive kynurenines. Int J Mol Sci. 2020;21(7):2431. 23. Possin KL. Visual spatial cognition in neurodegenerative disease. Neurocase. 2010;16(6):466-87. 24. Grossman M, Irwin DJ. The Mental Status Examination in patients with suspected dementia. Continuum (Minneap Minn). 2016;22(2 Dementia):385-403. 25. Risacher SL, WuDunn D, Tallman EF, et al. Visual contrast sensitivity is associated with the presence of cerebral amyloid and tau deposition. Brain Commun. 2020;2(1):fcaa019. 26. Kim HJ, Ryou JH, Choi KT, et al. Deficits in color detection in patients with Alzheimer disease. PLoS One. 2022;17(1):e0262226. 27. Thomas D, Thomas R, Muliyil JP, George R. Role of frequency doubling perimetry in detecting neuro-ophthalmic visual field defects. Am J Ophthalmol. 2001;131(6):734-41. 28. Grover S, Amitava AK, Kumari N. One glasses too many: a case report of Benson’s syndrome. Indian J Ophthalmol. 2015;63(3):277-9. 29. Asanad S, Felix CM, Fantini M, et al. Retinal ganglion cell dysfunction in preclinical Alzheimer’s disease: an electrophysiologic biomarker signature. Sci Rep. 2021;11(1):6344. 30. Moinuddin O, Khandwala NS, Young KZ, et al. Role of optical coherence tomography in identifying retinal biomarkers in frontotemporal dementia: a review. Neurol Clin Pract. 2021;11(4):e516-23. 31. Song A, Johnson N, Ayala A, Thompson AC. Optical coherence tomography in patients with Alzheimer’s disease: what can it tell us? Eye Brain. 2021;13:1-20. 32. Wagner SK, Romero-Bascones D, Cortina-Borja M, et al.; for UK Biobank Eye & Vision Consortium. Retinal optical coherence tomography features associated with incident and prevalent Parkinson disease. Neurology. 2023;101(16):e1581-93. 33. Costanzo E, Lengyel I, Parravano M, et al. Ocular biomarkers for Alzheimer disease dementia: an umbrella review of systematic reviews and meta-analyses. JAMA Ophthalmol. 2023;141(1):84-91. 34. Mohanty D, Hay KR, Berkowitz S, et al. Clinical implications of photophobia in progressive supranuclear palsy. Clin Park Relat Disord. 2021;4:100097. 35. Ungureanu L, Irincu L, Diaconu S, et al. Diplopia in movement disorders: a systematic review of the literature. J Pers Med. 2024;14(3):270. 36. Nagino K, Sung J, Oyama G, et al. Prevalence and characteristics of dry eye disease in Parkinson’s disease: a systematic review and meta-analysis. Sci Rep. 2022;12(1):18348. 37. Al-Janahi E, Ponirakis G, Al Hamad H, et al. Corneal nerve and brain imaging in mild cognitive impairment and dementia. J Alzheimers Dis. 2020;77(4):1533-43. 38. Romaus-Sanjurjo D, Regueiro U, López-López M, et al. Alzheimer’s disease seen through the eye: ocular alterations and neurodegeneration. Int J Mol Sci. 2022;23(5):2486. 39. Marchesi N, Fahmideh F, Boschi F, Pascale A, Barbieri A. Ocular neurodegenerative diseases: interconnection between retina and cortical areas. Cells. 2021;10(9):2394. 40. Shang X, Zhu Z, Huang Y, et al. Associations of ophthalmic and systemic conditions with incident dementia in the UK Biobank. Br J Ophthalmol. 2023;107(2):275-82. 41. Lee CS, Gibbons LE, Lee AY, et al. Association between cataract extraction and development of dementia. JAMA Intern Med. 2022;182(2):134-41. 42. Chellappa SL, Bromundt V, Frey S, et al. Association of intraocular cataract lens replacement with circadian rhythms, cognitive function, and sleep in older adults. JAMA Ophthalmol. 2019;137(8):878-85. 43. Agarwal P, Leurgans SE, Agrawal S, et al. Association of Mediterranean-DASH intervention for neurodegenerative delay and Mediterranean diets with Alzheimer disease pathology. Neurology. 2023;100(22):e2259-68. 44. Zhang L, Xu H, Ding N, et al. Beneficial effects on brain micro-environment by caloric restriction in alleviating neurodegenerative diseases and brain aging. Front Physiol. 2021;12:715443. 45. Liu Y, Yan T, Chu JMT, et al. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab Invest. 2019;99(7):943-57. 46. Bássoli RMF, Audi D, Ramalho BJ, et al. The effects of curcumin on neurodegenerative diseases: a systematic review. J Herb Med. 2023;42(3):100771. 47. Wang W, Li Y, Meng X. Vitamin D and neurodegenerative diseases. Heliyon. 2023;9(1):e12877. 48. Sala-Vila A, Tintle N, Westra J, Harris WS. Plasma omega-3 fatty acids and risk for incident dementia in the UK Biobank Study: a closer look. Nutrients. 2023;15(23):4896. |

