Eyelids & Ocular SurfaceThe November 2024 Review of Optometry issue showcases five articles on eyelid & ocular surface conditions, from MGD to scleritis. Read about what to do when dry eye patients aren’t responding to treatment, corneal AEs caused by systemic meds, innovative MGD treatments, managing ocular cellulitis and more. Check out the other articles featured in this issue: |
Managing dry eye conditions is a nuanced task that demands personalized treatment plans to effectively address the unique symptoms of each patient. Through meticulous diagnostic testing and careful interpretation of the results, optometrists can prescribe therapeutics that are most likely to achieve the best clinical outcomes. Nevertheless, successful results are not always guaranteed, and treatment plans may require ongoing adjustments. Suboptimal therapeutic outcomes should prompt the clinician to consider whether the correct diagnosis was made and conduct further testing to confirm.
While practitioners vary on their specific protocols for troubleshooting suboptimal outcomes in dry eye care, below I’ll share my approach and detail the various signs and symptoms of numerous dry eye differentials to keep in mind.
Reviewing the History
When a dry eye patient shows an inadequate treatment response, the first place to start is a comprehensive review of the patient’s medical history using a detailed questionnaire accompanied by a verbal discussion to ensure thorough understanding and accuracy. During the evaluation, meticulously note the recent symptoms experienced by the patient. Critical questions to explore include:
What symptoms are present, and what triggers them?
When do these symptoms intensify?
What is the patient’s current treatment plan?
Are there any therapies that have been attempted but failed?
Is there a history of medication use, autoimmune disorders or previous eye or eyelid surgeries or injuries?
It is also important to discuss the patient’s current systemic medications. Among the top 100 best-selling systemic drugs in the United States, about 22% disclose dry eye as a potential side effect. This includes common systemic meds such as antihistamines, antidepressants and isotretinoin. Topical ophthalmic agents, particularly those containing benzalkonium chloride (BAK), should also be considered when reviewing a patient’s medical history.
Certain lifestyle factors can also cause or exacerbate symptoms of dry eye. For example, does the patient work in a high-stress environment or consume a diet loaded with pro-inflammatory foods such as processed meats, high-fat dairy products and refined sugars? According to research presented in the Tear Film and Ocular Surface Society (TFOS) Nutrition Report, dietary intake undoubtedly affects the ocular surface. A notable percentage of the American diet is composed of processed food, introducing harmful ingredients to the body that can disrupt the gut microbiome and ultimately the ocular surface. Additionally, these processed foods and chemicals contribute to systemic disorders such as obesity and heart disease, which can lead to deficiencies in nutrients that are important to maintaining ocular surface health.2
Each of these aforementioned factors is crucial for understanding the full scope of a patient’s condition and formulating an effective care plan.
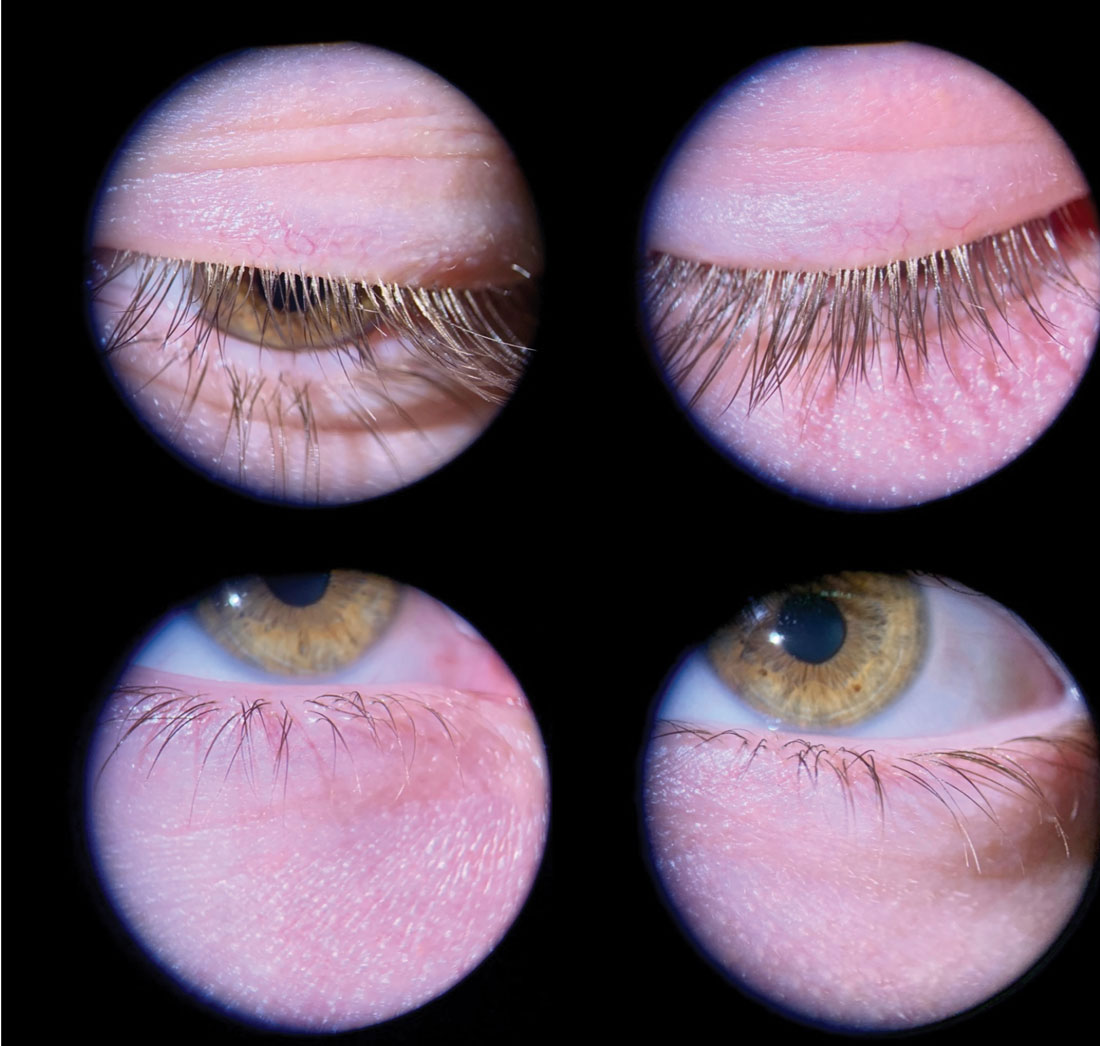 |
Fig. 1. Even mild Demodex blepharitis should be caught and treated early to prevent progression. This young male patient has collarettes at the lash bases and mild flaking. Photo: Andrew McLeod, OD, and Amy Nau, OD. Click image to enlarge. |
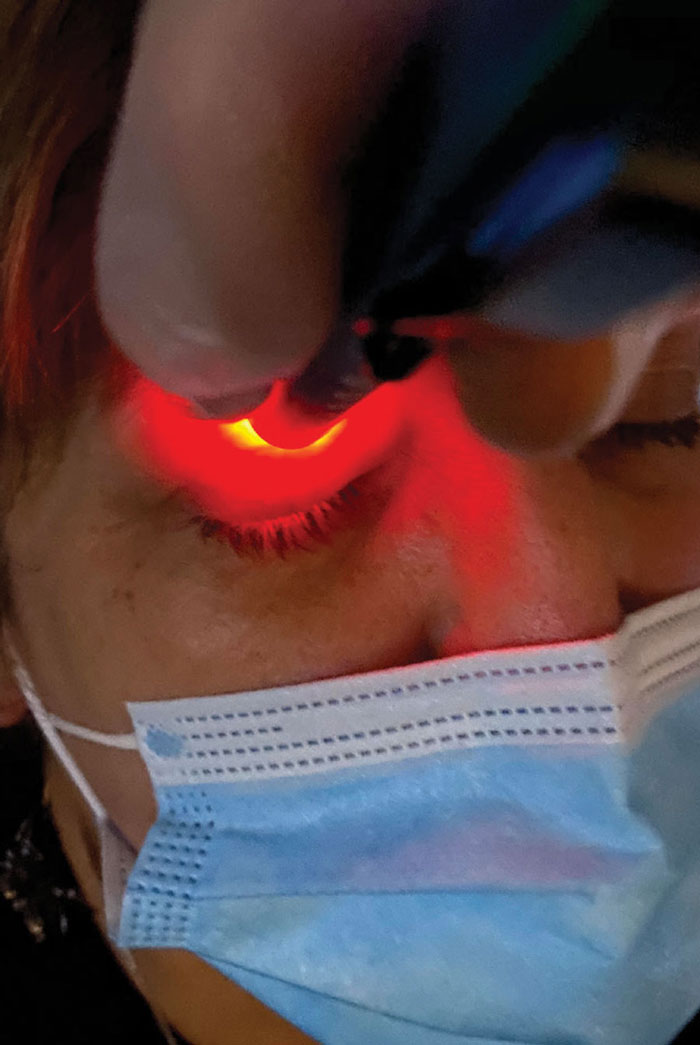 |
Fig 2. The Korb-Blackie light test can help detect incomplete lid seal, an underdiagnosed condition estimated to affect 61% of dry eye patients. Photo: Doug Devries, OD. Click image to enlarge. |
Clinical Examination
Every clinician has a unique battery of tests they perform during ocular surface examinations; for me, this consists of tear osmolarity, meibography, noninvasive tear break-up time (NiTBUT), corneal nerve sensitivity testing and fluorescein and lissamine green staining. Additionally, on slit lamp examination, I start by examining closed eyelids and lashes and taking note of any lid seal or laxity issue or Demodex collarettes (Figure 1). I closely examine the open eyelid margin looking for telangiectasia or irregularities, which may include keratin/debris buildup or saponification. I manually push on the meibomian glands to assess their secretions. I also visually assess tear film quality, corneal staining following fluorescein dye instillation and conjunctival staining or anomalies, such as conjunctivochalasis.
The key to finding the ideal treatment for any given patient’s dry eye symptoms is to trust the clinical test findings. Osmolarity and NiBUT will determine whether it’s a dry eye problem or diagnosis that presents with masquerading dry eye symptoms like eye irritation, soreness and fatigue. From there, vital dye stain can help narrow down the diagnosis.
Be sure to use diagnostic testing and clinical findings to formulate a plan that makes sense in light of the results. Most dry eye arises from a combination of evaporative and aqueous-deficient mechanisms, with a majority of cases relating to meibomian gland dysfunction. Managing all components of dry eye—not just the most conspicuous—will ensure successful treatment and symptomatic relief.
Targeted Treatments
Once you have confidently identified the etiology of a patient’s dry eye disease (DED), you can determine the appropriate intervention to address their clinical signs and symptoms.
Evaporative dry eye treatments should address meibomian gland obstruction, lid margin biofilms, inflammation and the quality of the tear film itself. Obstruction can be improved promptly with thermal meibomian gland expression technologies and improved long-term by recommending oral supplements. Lid margin biofilms can be addressed with blepharoexfoliation techniques and management of concurrent Demodex. Inflammation contributing to evaporative DED tends to respond well with different light therapies, such as intense pulsed light or low-level light therapy, as well as topical immunomodulators and anti-inflammatories.3 Lastly, in evaporative DED cases, tear film stability can be improved with pharmaceuticals such as perfluorohexyloctane or in-office irrigation techniques (more on this later).
Aqueous-deficient dry eye treatments should aim to mitigate inflammation and increase tear volume. Some therapy options include a short course of steroids, punctal plugs, neurostimulation, amniotic membranes, autologous serum and repository corticotropin injection.
Mucin-deficient DED may improve from preservative-free lubricating drops, topical vitamin A, topical corticosteroid drops, as well as returning the tear film to homeostasis.
This is an admittedly long list of treatment options and not every practitioner will be well-equipped to offer them all. The individual OD should reach for the most accessible options first while striving to incorporate others into the practice over time. The most important thing is to match the intervention to the specific cause of the patient’s symptoms.
Once the patient completes the initially prescribed treatment plan, instruct them to return to the office several weeks later for a progress evaluation. Follow-up ensures the patient is compliant and able to obtain the prescribed medications. At this appointment, the patient questionnaire should be repeated, along with the clinical examination.
The success of dry eye treatment should be measured based on improvements in diagnostic testing and clinical and patient symptoms. The biggest challenge is when patients don’t improve as expected. In these scenarios, repeat your diagnostic testing and review clinical findings with a fine-tooth comb. Was the initial treatment plan addressing the correct disease type? If so, then it is time to consider the contribution of the palpebral conjunctiva and fornix to ocular surface health and tear stability; it’s also wise to consider potential dry eye masqueraders.
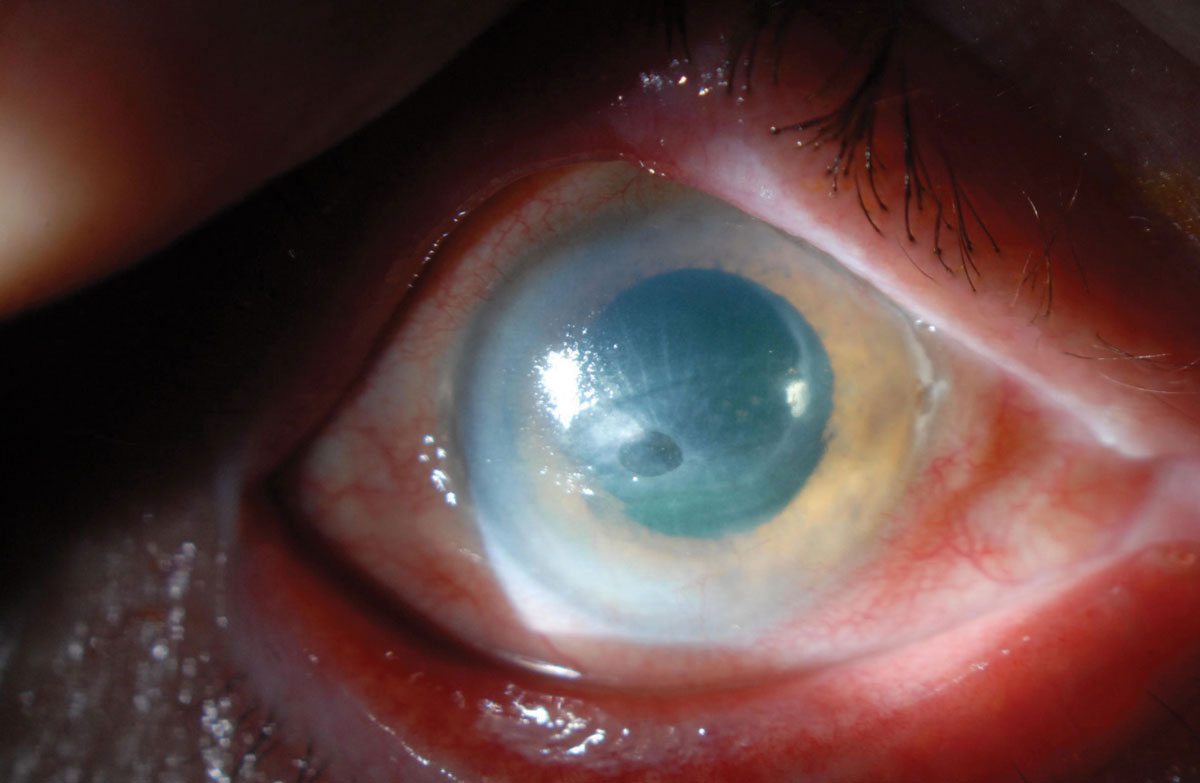 |
|
Fig. 3. While neurotrophic keratitis is often overlooked in its early stages, sooner detection is key to elude complications such as corneal melt. Photo: Zeba A. Syed, MD. Click image to enlarge. |
Ocular Surfaces to Re-evaluate
Sometimes, the greatest solution is dilution. Rinsada is an irrigating eyelid retractor that targets the palpebral conjunctiva and fornix using high-pressure irrigation of sterile saline. The concept of rinsing the ocular surface and surrounding palpebral conjunctiva and fornix is to reduce the inflammatory load.4 The significance of this design is the direction of fluid, which targets this anatomical area in ways that no other procedure or treatment can provide. (Editor’s note: the author is a consultant to the manufacturer of this device.)
Randomized controlled trial data for Rinsada illustrated a notable reduction of 72% of MMP-9 within the first three hours after a rinse. Some patients even converted to negative MMP-9 testing for up to 12 weeks.5
If a patient does not respond to current therapies, it may be that the involvement of the palpebral conjunctiva and fornix is so great that it is preventing homeostasis of the ocular surface. By reducing this inflammatory, pollutant and microparticulate load through eye irrigation and simultaneous lid eversion, patients may experience immediate relief. More importantly, the treatment can reset the surface by removing inflammation and allergens.
Beyond the palpebral conjunctiva and fornix, attentive assessment of the tear film and ocular surface will also help guide which pharmaceutical or other therapeutic options may be beneficial. For instance, a reduced overall tear lake may benefit from varenicline nasal spray (one spray per nostril BID), while cyclosporine, loteprednol or lifitegrast can help reduce ocular inflammation.6,7
DED Masqueraders
Before initiating treatment or when a patient doesn’t respond to the prescribed therapy, it is important to be cognizant of the many alternate diagnoses that disguise themselves by causing dry eye symptoms. Here are a few to keep in mind:
Lid laxity/floppy eyelid. The mechanism of a blink is intricate as well as critical to the mechanism of how tears are made. Looseness of eyelids can be a contributing factor to dry eye symptoms. Floppy eyelid syndrome is an underdiagnosed disease commonly associated with obesity, sleep apnea and keratoconus.8 There is no standard test to diagnose this condition, but a physical examination involving flipping of the upper eyelid should be the clinical indicator. If the lid flips on its own or is easily everted without much manipulation, be suspicious of floppy lid syndrome. If you think of it in a physical context, when the lid everts, the eyeball is left to dry through the night.
Some simple nighttime routines may provide patients with symptomatic relief. Management can be as easy as using a nighttime ointment, shield or mask or taping the eyelids shut during sleep. In severe cases, surgical intervention may be warranted.
Incomplete lid seal. This issue presents in roughly 61% of dry eye patients.9 A diagnosis of incomplete lid seal can be accomplished with a Korb-Blackie light test, where an inferiorly placed transilluminator is used to view escaping light in a closed eye (Figure 2). A quicker way to diagnose this condition is to ask the patient how their eyes feel in the morning. If their symptoms are worse in the morning, be extremely suspicious of this differential. Incomplete lid seal causes nocturnal evaporative stress to the ocular system, presenting in the mornings as moderate to severe dry eye symptoms.
An important reminder for clinicians is to check not only for inferior corneal staining but also for inferior conjunctival staining. Due to Bell’s phenomenon during the nighttime, sometimes the staining will not be present on the cornea.
Although lagophthalmos has gained some attention as an underdiagnosed finding, incomplete lid seal is significantly more common than true lagophthalmos.
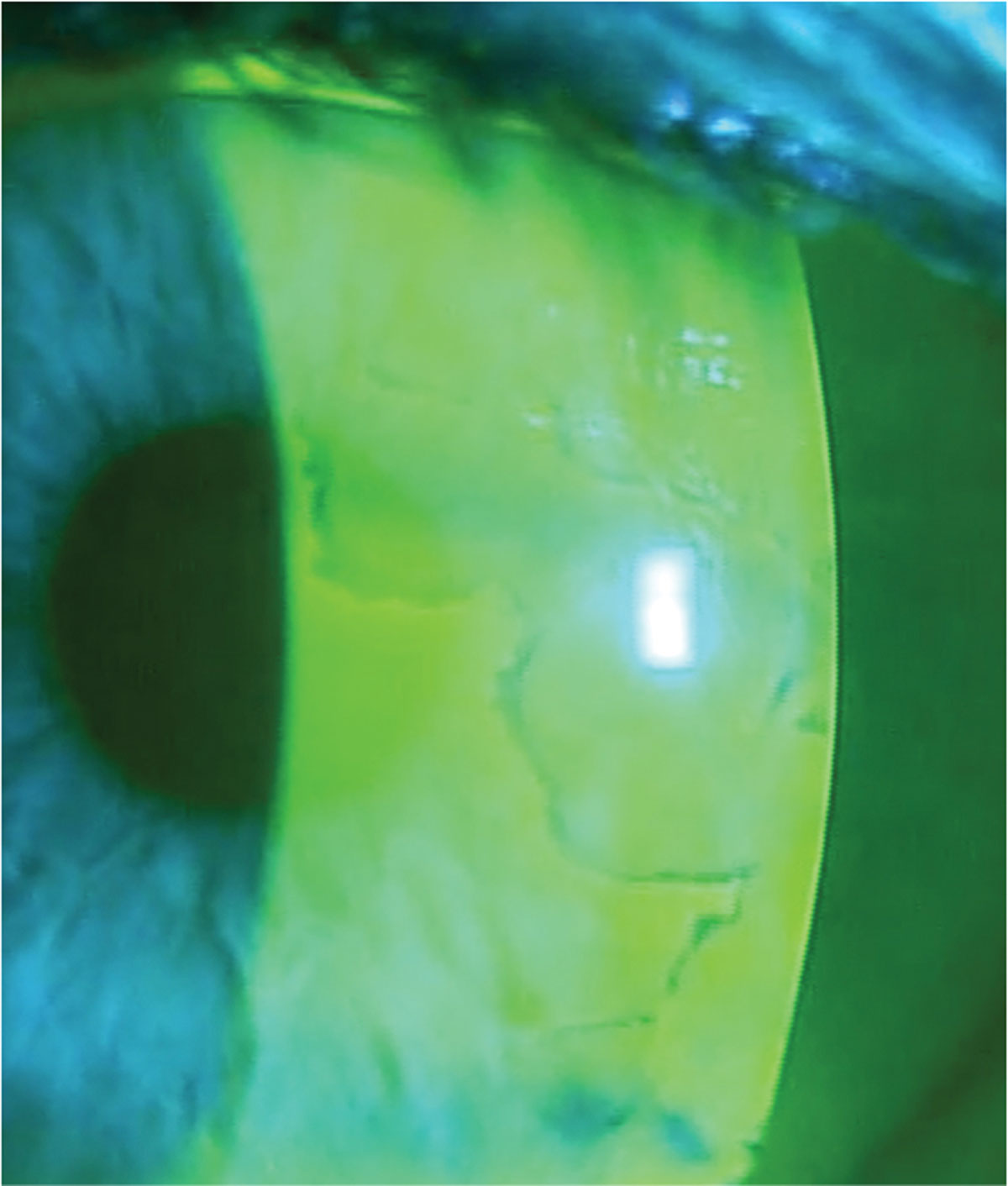 |
Fig. 4. Using sodium fluorescein dye at the slit lamp helps delineate dots and fingerprint patterns associated with EBMD. Photo: Mile Brujic, OD, and David Kading, OD. Click image to enlarge. |
Demodex. Especially in mild cases, Demodex blepharitis can be easily overlooked or not addressed, though it can cause significant discomfort for patients. When examining lashes for collarettes at the slit lamp, use a higher magnification and have the patient look down. Pulling up on the upper lids will also expose the roots of the lashes. The change from low to high magnification and redirection of patient gaze has helped me identify a significantly larger number of Demodex patients.
Do not underestimate the discomfort Demodex can cause. Demodex mites feast on epithelial cells at the hair follicles. Their claws can cause small abrasions, leading to epithelial hyperplasia.10 Additionally, Demodex brevis has been linked to meibomian gland dysfunction and even meibomian gland loss.11 It can respond well to a course of lotilaner (Xdemvy, Tarsus Pharmaceuticals), dosed BID for six weeks.
Neurotrophic keratitis (NK). Any injury or systemic condition affecting corneal innervation can lead to NK.12 This degenerative disease is caused by impairment of corneal sensory innervation. The hallmark clinical finding is decreased or absent corneal sensation, which is easy to test in-clinic with a cotton wisp, the corner of a tissue or dental floss. When corneal nerve sensitivity is reduced, epithelial breakdown and impaired corneal healing will occur.13 This can ultimately progress to corneal ulceration, melting and perforation (Figure 3).
As is the case with many of these dry eye masqueraders, it is easier to overlook NK in its early stages, but persistent dry eye symptoms should raise your suspicion of this differential. Treatment options for NK consist of biologics like cenergermin-bkbj (Oxervate, Dompé) cryopreserved amniotic membranes and autologous serum.14
Epithelial basement membrane dystrophy (EBMD). This condition occurs due to an atypical thickening of the basement membrane that extends into the corneal epithelium. As corneal epithelial cells mature, they move towards the anterior surface. These cells become trapped between the layers and form thickened basement membranes that can present in a pattern resembling fingerprints (Figure 4). EBMD is the most common corneal dystrophy, affecting about 76% of the population over age 50.15 These patients will often present with fluctuating vision and foreign body sensation; they may also have a history of recurrent corneal erosion.
To diagnose EBMD, assess the cornea under slit lamp examination looking for epithelial microcysts in the form of irregular grayish-white opacities (“dots”) and whirling defects (“fingerprints”).16 Depending on the severity and visual significance of EBMD, procedural intervention may be appropriate. This may include superficial keratectomy with a diamond burr polishing, anterior stromal puncture or phototherapeutic keratectomy. These procedures will reset the epithelium and promote adherence of the epithelium to the basement membrane.17
 |
|
Fig. 5. Depending on the severity of conjunctivochalasis, treatment options consist of lubricating drops, topical corticosteroids, antihistamines and even surgery when warranted. Photo: John P. Herman, OD. Click image to enlarge. |
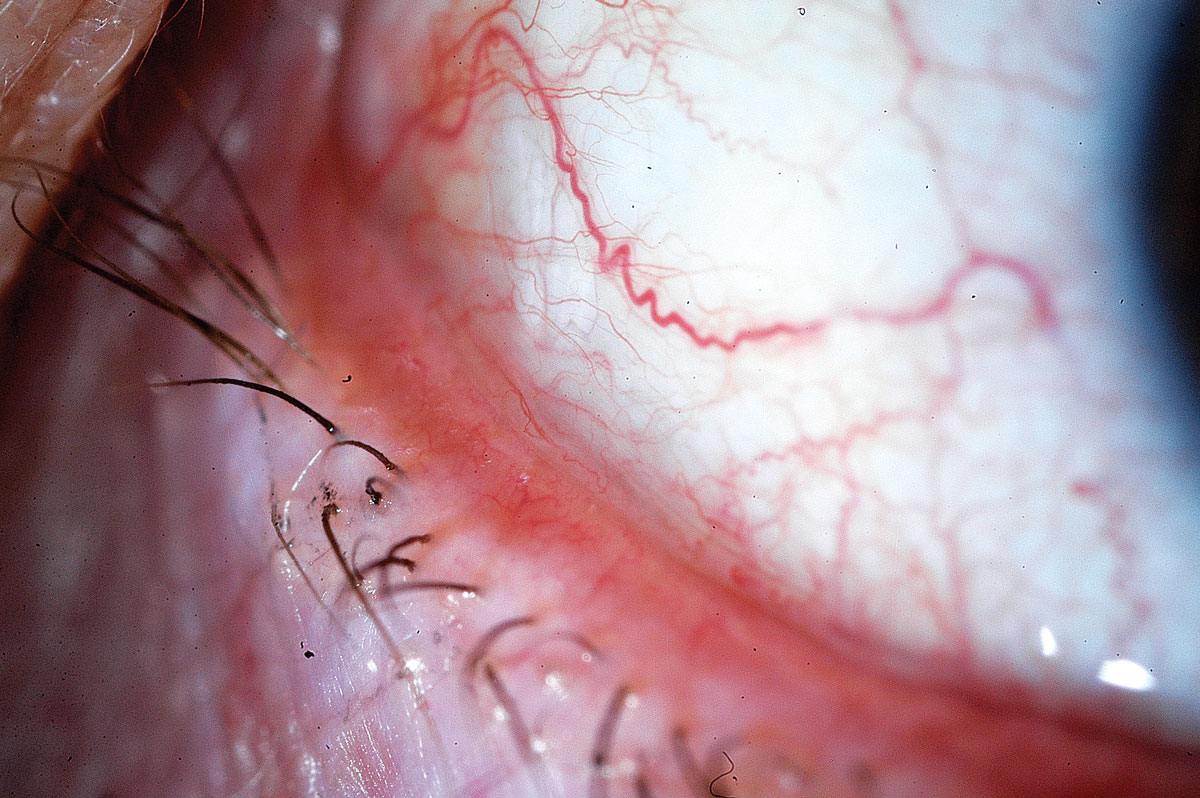
Conjunctivochalasis (CCH). Minor clinical findings can have disproportionately greater symptomatic impact in this fairly common condition (Figure 5). CCH is caused by a degeneration of Tenon’s capsule and commonly presents as folds sitting on inferior bulbar conjunctiva, which can interfere with tear drainage, tear meniscus and tear lake.18 Additionally, when patients with CCH have their conjunctiva cultured, the fibroblasts show a high level of MMPs with increased inflammation and oxidative stress on the tears.19 In mild cases of CCH, lubricants may provide symptomatic relief. Certain ingredients like isotonic glycerol and sodium hyaluronate are proposed to help decrease disease severity.20
Topical corticosteroids may be appropriate, as they help reduce inflammation, as well as antihistamines, which can minimize mechanical eye rubbing by the patient. Consider more aggressive surgical approaches when topical therapies are insufficient.
Superior limbic keratoconjunctivitis (SLK). Characterized by chronic inflammation of the superior bulbar conjunctiva, the superior limbus and the tarsal conjunctiva, this condition is associated with systemic thyroid disease (Figure 6).21 Patients often present with symptoms of foreign body sensation (71.1%) and burning (68.9%).22 The etiology and pathophysiology of SLK are unknown, though it is believed that the chronic inflammation and physical rubbing cause thickening of the conjunctiva, which continues to propagate the inflammatory response.23 While there is no recommended first-line treatment for SLK, most therapies aim to reduce inflammation and manage symptoms.
Asthenopia. Many symptoms of binocular vision anomalies and trigeminal dysphoria may present similarly to dry eye symptoms: irritated eyes worsening by the end of the day, eye strain and neck and shoulder pain.24 A cover test should be performed to support or negate the diagnosis.
Asthenopia patients’ symptoms usually worsen at the end of the day and will have a normal osmolarity reading. Adding a question about headache and neck strain to the patient questionnaire may also help indicate trigeminal dysphoria. Treatment for binocular vision and trigeminal dysphoria may consist of spectacle lenses that include prism or vision therapy.
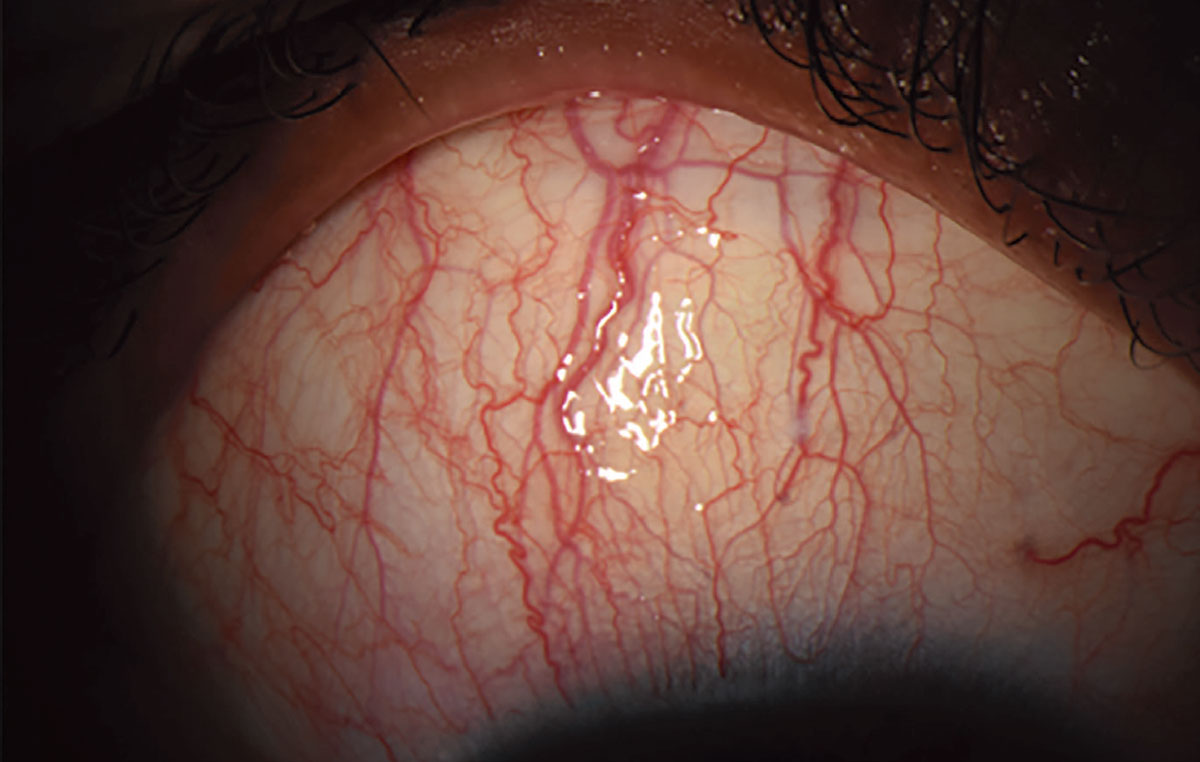 |
Fig. 6. Most patients with SLK present with symptoms of foreign body sensation and burning. Photo: Sezen Karakus, MD. Click image to enlarge. |
Takeaways
Treating DED should be approached systematically. Using a battery of tests and exam findings to diagnose and initiate treatment plans can ensure clinical improvement and symptomatic relief. In cases where a patient doesn’t seem to be improving, review the testing and slit lamp imaging and be suspicious of the various diagnoses that can masquerade as DED.
Dr. Noh owns Noh Eyes, a cold-start dry eye specialty practice she opened two years ago that focuses solely on the diagnosis and treatment of dry eye disease. She consults for Dompé, Rinsada and Physician Recommended Nutriceuticals and is on the advisory board for Tarsus Pharmaceuticals.
1. PDR Staff. Physicians desk reference. 64th ed. Montvale, NJ, USA: PDR Network, LLC; 2010. 2. Markoulli M, Ahmad S, Arcot J, et al. TFOS lifestyle: impact of nutrition on the ocular surface. Ocul Surf. 2023;29:226-71. 3. Green-Church KB, Butovich I, Willcox M, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid–protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52(4):1979-93. 4. Kim A, Postnikoff CK, Nichols KK. Nonpharmaceutical eye wash may reduce matrix metalloprotease-9 (MMP-9) in dry eye. Invest Ophthalmol Vis Sci. 2020;61(7):100. 5. Mayer N, Kondapalli SSA, Venkateswaran N, et al. The efficacy of an irrigating eyelid retractor-facilitated ocular rinse on MMP-9 expression and dry eye disease. Adv Ophthalmol Pract Res. 2024;4(3):142-6. 6. Yu MD, Park JK, Kossler AL, Saeed HN. Stimulating tear production: spotlight on neurostimulation. Clin Ophthalmol. 2021;15:4219-26. 7. Gao J, Sana R, Calder V, et al. Mitochondrial permeability transition pore in inflammatory apoptosis of human conjunctival epithelial cells and T cells: effect of cyclosporin A. Invest Ophthalmol Vis Sci. 2013;54(7):4717-33. 8. Beis PG, Brozou CG, Gourgoulianis KI, et al. The floppy eyelid syndrome: evaluating lid laxity and its correlation to sleep apnea syndrome and body mass index. ISRN Ophthalmol. 2012;2012:650892. 9. Korb D, Blackie C, Nau A. Prevalence of compromised lid seal in symptomatic refractory dry eye patients and asymptomatic patients. Invest Ophthalmol Vis Sci. 2017;58:2696. 10. Luo X, Li J, Chen C, et al. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea. 2017;36(Suppl 1):S9-14. 11. Zhang AC, Muntz A, Wang MTM, et al. Ocular Demodex: a systematic review of the clinical literature. Ophthalmic Physiol Opt. 2020;40:389-432. 12. Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571-9. 13. Mastropasqua L, Massaro-Giordano G, Nubile M, Sacchetti M. Understanding the pathogenesis of neurotrophic keratitis: the role of corneal nerves. J Cell Physiol. 2017;232(4):717-24. 14. Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111(6):1115-20. 15. Werblin TP, Hirst LW, Maumenee IH. Prevalence of map-dot-fingerprint changes in the cornea. Br J Ophthalmol. 1981;65(6):401-9. 16. Kobayashi A, Yokogawa H, Sugiyama K. In vivo laser confocal microscopy findings in patients with map-dot-fingerprint (epithelial basement membrane) dystrophy. Clin Ophthalmol. 2012;6:1187-90. 17. Miller DD, Hasan SA, Simmons NL, Stewart MW. Recurrent corneal erosion: a comprehensive review. Clin Ophthalmol. 2019;13:325-35. 18. Cheng AM, Yin HY, Chen R, et al. Restoration of fornix tear reservoir in conjunctivochalasis with fornix reconstruction. Cornea. 2016;35(6):736-40. 19. Wang Y, Dogru M, Matsumoto Y, et al. The impact of nasal conjunctivochalasis on tear functions and ocular surface findings. Am J Ophthalmol. 2007;144(6):930-7. 20. Kiss HJ, Németh J. Isotonic glycerol and sodium hyaluronate containing artificial tear decreases conjunctivochalasis after one and three months: a self-controlled, unmasked study. PLoS One. 2015;10(7):e0132656. 21. Cher I. Clinical features of superior limbic keratoconjunctivitis in Australia: a probable association with thyrotoxicosis. Arch Ophthalmol. 1969;82(5):580-6. 22. Mendoza-Adam G, Rodríguez-García A. Superior limbic keratoconjunctivitis (SLK) and its association to systemic diseases. Rev Mex Oft. 2013;87(2):93-9. 23. Nelson JD. Superior limbic keratoconjunctivitis (SLK). Eye (Lond). 1989;3(Pt 2):180-9. 24. Karpecki PM. The dry eye misalignment. Rev Optom. August 15, 2018. |

